Tebbe methylenation
The Tebbe methylenation , or Tebbe reaction , is a chemical reaction from the field of organic chemistry . It is used for methylenation , i.e. the introduction of a methylene group . Carbonyl components such as aldehydes , ketones and esters , but also amides, are used as starting materials . Titanium-containing complexes such as the Tebbe , Petasis or Lombardo reagent are used as reagents . The reaction is named after its developer, the American chemist Frederick Nye Tebbe .
The Tebbe methylenation is an alternative to the Wittig reaction .
mechanism
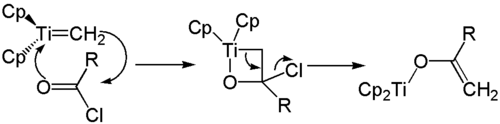
The reactive Schrock carbene is initially formed from the Tebbe reagent through the action of bases (for example pyridine ) . This added first to the carbonyl compound to form an oxa - derivative of a Titana cyclobutane . This then opens, releasing the alkene and a titanium-oxygen species.
However, there is evidence that the mechanism is not a purely concerted process as in olefin metathesis , but that ionic species occur as reaction intermediates.
The formation of the deformylated main product cannot be explained by a purely concerted mechanism.
If carboxylic acid halides are used as the carbonyl component, the four-membered ring opens with elimination of a halide ion . An enolate is formed which is stabilized by the bound titanium complex. The enolate formed can now be converted to an enol ether , for example with an alkyl halide .
operation area
The Tebbe reagent only allows the introduction of methylene groups, in contrast to the Wittig reaction , with which a wide range of residues can be introduced. An advantage over the Wittig reaction lies in the possibility of using esters and amides which cannot be used in Wittig reactions.
Significantly higher yields are also achieved by a Tebbe methylenation in the case of sterically hindered carbonyls. Chiral carbon atoms in the α-position to the carbonyl do not racemize during a Tebbe reaction.
The use of the Petasis reagent results in an expansion of the area of application. This allows the introduction of a wider range of residues.
Olefin metathesis properties have been described for the Tebbe reagent and an olefin metathesis with formation of an enol ether takes place after the olefination reaction of an ester.
Individual evidence
- ^ FN Tebbe, GW Parshall and GS Reddy: Olefin homologation with titanium methylene compounds , J. Am. Chem. Soc. 1978 , 100 , 3611-3613.
- ↑ SH Pine, Org.React. 1993 , 43 , 1.
- ↑ I. Beadham, J. Micklefield, J. Curr. Org. Syn. 2005 , 2 , 231-250.
- ^ Kai Oesterreich, Dissertation University of Hohenheim 2004 , p. 61 ISBN 3-8325-0568-7 .
- ↑ A. Marra, J. Esnault, A. Veyrieres, P. Sinay: Isopropenyl glycosides and congeners as novel classes of glycosyl donors: theme and variations , J. Am. Chem. Soc. 1992 , 114 , 6354-6360.
- ↑ KC Nicolaou, MHD Postema: In J. Am. Chem. Soc. 1996 , 118 , 1565-1566.
- ↑ KC Nicolaou, MHD Postema, EW Yue, A. Nadin: In J. Am. Chem. Soc 1996 , 118 , 10335-10336.