Zeise salt
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
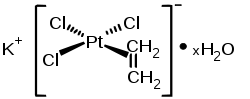
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Zeise salt | ||||||||||||||||||
| other names |
Potassium trichloridoethylene platinum (II) hydrate |
||||||||||||||||||
| Molecular formula | K [PtCl 3 (C 2 H 4 )] | ||||||||||||||||||
| Brief description |
yellow solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 368.61 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
2.88 g cm 3− |
||||||||||||||||||
| Melting point |
220 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
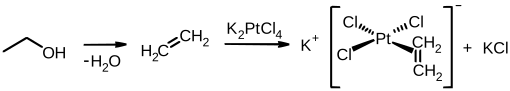
The Zeise salt is a platinum complex with the formula K [PtCl 3 (C 2 H 4 )] · H 2 O. It was discovered in 1825 or 1827 by the Danish chemist William Christopher Zeise and is considered one of the first synthesized organometallic compounds.
Presentation and extraction
Zeise obtained the complex by boiling potassium tetrachloridoplatinate (II) (K 2 [PtCl 4 ]) in ethanol . During this process, water is split off from ethanol with the formation of ethene . This then coordinates to the platinum center and forms the Zeise salt.
Zeise postulated at the time that the resulting salt contained ethene. However, this could only be confirmed decades later when potassium tetrachloridoplatinate (II) was reacted with ethene and the same salt was obtained.
structure
In the Zeise salt, the central platinum ion is coordinated in a square-planar manner. In the solid state, the ethylene ligand is perpendicular to this plane, but in solution it rotates around the σ bond to the metal ion. The hydrogen atoms are not in the same plane as the carbon atoms as in ethene, but are a little further away from the platinum. The C – C bond is slightly longer than that in ethene. By elimination of potassium chloride which can dimer of Zeise salt are obtained.
See also
Individual evidence
- ↑ a b c d e data sheet Potassium trichloro (ethylene) platinate (II) hydrate from Sigma-Aldrich , accessed on April 25, 2011 ( PDF ).
- ↑ External identifiers of or database links to potassium trichloro (ethylene) platinum (II) : CAS number: 12012-50-9, EC number: 234-577-7, ECHA InfoCard: 100.031.421 , PubChem : 197135 , ChemSpider : 21241825 , Wikidata : Q90695854 .
- ↑ a b c Richard A. Love, Thomas F. Koetzle, Graheme JB Williams, Lawrence C. Andrews, Robert Bau: Neutron diffraction study of the structure of Zeise's salt, KPtCl 3 (C 2 H 4 ) · H 2 O . In: Inorganic Chemistry . tape 14 , no. 11 , 1975, p. 2653-2657 , doi : 10.1021 / ic50153a012 .
- ↑ Christoph Elschenbroich: Organometallchemie . 6th edition, Teubner, Wiesbaden 2008, ISBN 978-3-8351-0167-8 , p. 367 ( limited preview in the Google book search).
- ↑ William Christopher Zeise : From the effect between platinum chloride and alcohol, and from the resulting new substances . In: Johann Christian Poggendorff, Berlin (Ed.): Annals of Physics and Chemistry . tape 97 , no. 4 . Johann Ambrosius Barth, Leipzig, 1831, p. 497-541 , doi : 10.1002 / andp.18310970402 ( online on the pages of Gallica - Bibliothèque numérique der Bibliothèque nationale de France - Volume 21 of Poggendorff's Annals).
- ↑ William Christopher Zeise : About the mutual decomposition taking place between platinum chloride and alcohol, and the resulting new bodies . In: Franz Wilhelm Schweigger-Seidel, Halle (Hrsg.): Journal for Chemistry and Physics . tape 62 , no. 4 , 1831, p. 393–441 ( online on the pages of archive.org - The Internet Archive - at the same time: New Yearbook of Chemistry and Physics of the Pharmaceutical Institute in Halle).
- ↑ William Christopher Zeise : About the mutual decomposition taking place between platinum chloride and alcohol, and the resulting new bodies (continued) . In: Franz Wilhelm Schweigger-Seidel, Halle (Hrsg.): Journal for Chemistry and Physics . tape 63 , no. 2 , 1831, p. 121–135 ( online on the pages of archive.org - The Internet Archive - simultaneously: New Yearbook of Chemistry and Physics of the Pharmaceutical Institute in Halle).
- ↑ Karl Birnbaum: About the compounds of ethylene and its homologues with platinum chloride . In: Justus Liebig's Annals of Chemistry . tape 145 , no. 1 , 1868, p. 67-77 , doi : 10.1002 / jlac.18681450115 ( online on the pages of archive.org - The Internet Archive ).
- ↑ M. Black, RHB corn and PG Owston: The crystal and molecular structure of Zeise's salt, KPtCl 3 · C 2 H 4 · H 2 O . In: Acta Crystallographica Section B . tape 25 , no. 9 September 1969, p. 1753-1759 , doi : 10.1107 / S0567740869004699 .
- ^ Christoph Elschenbroich : Organometallchemie 2002 Teubner Verlag.