1,2-dichloroethane
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | 1,2-dichloroethane | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 2 H 4 Cl 2 | |||||||||||||||||||||
| Brief description |
colorless, oily liquid with a chloroform-like odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 98.97 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
1.25 g cm −3 |
|||||||||||||||||||||
| Melting point |
−36 ° C |
|||||||||||||||||||||
| boiling point |
84 ° C |
|||||||||||||||||||||
| Vapor pressure |
|
|||||||||||||||||||||
| solubility |
poor in water (8.7 g l −1 at 20 ° C) |
|||||||||||||||||||||
| Refractive index |
1.4422 (25 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Authorization procedure under REACH |
of particular concern : carcinogenic ( CMR ); subject to approval |
|||||||||||||||||||||
| MAK |
|
|||||||||||||||||||||
| Global warming potential |
1 (based on 100 years) |
|||||||||||||||||||||
| Thermodynamic properties | ||||||||||||||||||||||
| ΔH f 0 |
−166.8 kJ / mol |
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
1,2-dichloroethane ( ethylene dichloride , EDC , formerly also chlorine ether ) is a colorless, flammable and poisonous liquid with a chloroform- like odor. This chemical compound is one of the chlorinated hydrocarbons .
history
1,2-Dichloroethane was first synthesized in 1794 by the four Dutch chemists Adriaan Paets van Troostwijk , Johan Rudolph Deiman , Nicolaas Bondt and Anthonie Lauwerenburgh by reacting ethene with chlorine ( oil from the Dutch chemists ).
Extraction and presentation
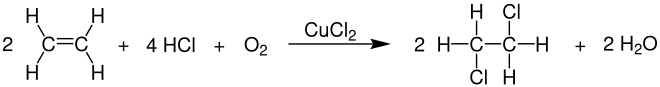
1,2-Dichloroethane is produced on an industrial scale by oxychlorination of ethene with hydrogen chloride and oxygen in the gas phase at temperatures of 200-360 ° C. and pressures of 1-9 bar. Copper (II) chloride is used as the catalyst , and iron (III) chloride is also used occasionally .
The catalyst support is arranged as a fixed or fluidized bed and can generally take place in any known type of reactor. Fluidized bed reactors are particularly preferred .
Another industrial process for the production of 1,2-dichloroethane is based on the direct chlorination of ethene . Metal halides such as iron (III) chloride are also used as a catalyst for this purpose.
properties
1,2-dichloroethane is a colorless, flammable liquid. The miscibility with water is very limited. As the temperature rises, the solubility of 1,2-dichloroethane in water increases or the solubility of water in 1,2-dichloroethane increases.
Solubilities between 1,2-dichloroethane and water temperature ° C 0 9.3 19.7 29.7 39.4 50.3 61.0 70.6 80.7 1,2-dichloroethane in water in% 0.82 0.77 0.72 0.81 0.98 1.06 1.08 1.13 1.06 Water in 1,2-dichloroethane in% 0.106 0.133 0.176 0.230 0.276 0.349 0.412 0.492
Safety-related parameters
1,2-dichloroethane forms highly flammable vapor-air mixtures. The compound has a flash point of 13 ° C. The explosion range is between 4.2% by volume (174 g / m 3 ) as the lower explosion limit (LEL) and 16% by volume (660 g / m 3 ) as the upper explosion limit (UEL). Correlating the explosion limits with the vapor pressure function results in a lower explosion point of 8 ° C. The limit gap width was determined to be 1.8 mm. This results in an assignment to explosion group IIA. The ignition temperature is 440 ° C. The substance therefore falls into temperature class T2.
use
Dichloroethane is used to make vinyl chloride . It is converted to vinyl chloride and hydrogen chloride by thermal elimination . 1,1,1-Trichloroethane is also produced from 1,2-dichloroethane . It is a good solvent and extractant. The 1,2-dichloroethane is used in paint strippers , as so-called scavengers in leaded fuels, solvents for resins ( synthetic resin / natural resin ) and asphalt and in bitumen .
It is also used on an industrial scale for the production of ethylene amines, especially ethylene diamine , by ammonolysis :
Decomposition by bacteria
Researchers at Ghent University have succeeded in isolating a bacterial strain that can completely break down the carcinogenic and toxic 1,2-dichloroethane within a few days. The scientists decided to name the strain Desulfitobacterium dichloroeliminans .
safety instructions
1,2-Dichloroethane is irritating, narcotic, mutagenic, carcinogenic and leads to organ damage ( liver , kidneys , blood ). It can also increase premature and dead birth rates. It leads to poisoning if swallowed and is carcinogenic . 1,2-dichloroethane is highly flammable. Dangerous gases can be produced when burned. Reactions with alkali metals can cause explosions. 1,2-dichloroethane is very harmful to the environment and hazardous to water ( water hazard class 3).
Individual evidence
- ↑ a b c d e f g h i j k l m n o p q r s Entry on 1,2-dichloroethane in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-154.
- ↑ Entry on 1,2-dichloroethane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on July 17, 2014.
- ↑ Entry in the register of substances subject to authorization of the European Chemicals Agency , accessed on July 17, 2014.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 107-06-2 or 1,2-dichloroethane ), accessed on September 14, 2019.
- ↑ G. Myhre, D. Shindell et al .: Climate Change 2013: The Physical Science Basis . Working Group I contribution to the IPCC Fifth Assessment Report. Ed .: Intergovernmental Panel on Climate Change . 2013, Chapter 8: Anthropogenic and Natural Radiative Forcing, pp. 24-39; Table 8.SM.16 ( PDF ).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-22.
- ↑ Entry on dichloroethane. In: Römpp Online . Georg Thieme Verlag, accessed on June 8, 2014.
- ↑ See also the term oil of the olefiant gas in Justus Liebig . See Albert Faulconer, Thomas Edward Keys: Chloroform. In: Foundations of Anesthesiology. 2 volumes, Charles C Thomas, Springfield (Illinois) 1965, Volume 1, pp. 442-481, here: p. 455.
- ^ A b Heiko Urtel, Henrik Junicke, Rupert Wagner, Volker Schuda: Process for the production of 1,2-dichloroethane. In: Google Patents. BASF SE, February 12, 2009, accessed March 14, 2019 .
- ^ A b R. M. Stephenson: Mutual Solubilities: Water-Ketones, Water-Ethers, and Water-Gasoline-Alcohols in J. Chem. Eng. Data 37 (1992) 80-95, doi : 10.1021 / je00005a024 .
- ^ A b c d E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ↑ Ann Maes, Hilde Van Raemdonck, Katherine Smith, Wendy Ossieur, Luc Lebbe, Willy Verstraete: Transport and Activity of Desulfitobacterium dichloroeliminans Strain DCA1 during Bioaugmentation of 1,2-DCA-Contaminated Groundwater. In: Environmental Science & Technology. 40, 2006, pp. 5544-5552, doi : 10.1021 / es060953i .