1,1,1-trichloroethane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
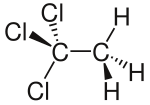
|
||||||||||||||||
| Wedge line formula to clarify the geometry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,1,1-trichloroethane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 3 Cl 3 | |||||||||||||||
| Brief description |
colorless liquid with an ethereal, chloroform-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 133.42 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.34 g cm −3 |
|||||||||||||||
| Melting point |
−30 ° C |
|||||||||||||||
| boiling point |
74 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
heavy in water (1 g l −1 at 25 ° C) |
|||||||||||||||
| Refractive index |
1.4366 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| Global warming potential |
193 (based on 100 years) |
|||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−177.4 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1,1,1-trichloroethane (old common name methylchloroform ) belongs to the chlorinated hydrocarbon family . It is isomeric to 1,1,2-trichloroethane .
Manufacturing
The chemical is made by chlorinating 1,1-dichloroethane or by adding hydrogen chloride to 1,1-dichloroethene .
properties
1,1,1-Trichloroethane is a colorless liquid with a typical ethereal odor (comparable to the odor of chloroform ). It is hardly miscible with water , i.e. hydrophobic and lipophilic and its vapors are heavier than air. Like other chlorinated hydrocarbons, it is unstable to light, air and especially moisture. Stabilization takes place by adding up to 6% dioxane or 2-butanol during manufacture. It reacts explosively with alkali , alkaline earth and other metal powders. Inadequately stabilized 1,1,1-trichloroethane attacks aluminum, and an explosive reaction with simultaneously present aromatics is possible.
Safety-related parameters
1,1,1-trichloroethane forms flammable vapor-air mixtures. The explosion range is between 9.5% by volume (529 g / m 3 ) as the lower explosion limit (LEL) and 15.5% by volume (860 g / m 3 ) as the upper explosion limit (UEL). With a minimum ignition energy of 4800 mJ, the connection is difficult to ignite. The ignition temperature is 490 ° C. The substance therefore falls into temperature class T1.
use
1,1,1-Trichloroethane is a starting material for the synthesis of polychlorinated organic compounds through addition reactions with unsaturated compounds.
It was used as a solvent in paints, adhesives , cutting oils and for cleaning metallic workpieces, glass, optical devices and machines in the textile industry . It can also be found in correction fluids. Tipp-Ex contains 1,1,1-trichloroethane as a solvent, but there is also a water-based variant because of the possible health risk. In the course of the detection of the ozone hole problem, its importance as a solvent has decreased.
safety instructions
Despite the lower toxicity than carbon tetrachloride , the vapors irritate the mucous membranes. Liver and kidney damage are possible through absorption into the body. Trichloroethane absorbed in gaseous form is almost completely excreted through the breath, a small part is metabolized in the body to trichloroacetic acid and trichloroethanol . If the MAK value is observed, fruit damage is very unlikely. Like many solvents, it has a harmful degreasing effect on the skin. It can irritate the airways, digestive tract, and eyes. Dizziness, headaches, drowsiness or loss of consciousness and other brain dysfunction may occur.
The chlorine atoms released by photochemical processes in the stratosphere can damage the ozone layer .
Individual evidence
- ↑ a b c d e f g h i j k l m n o Entry on 1,1,1-trichloroethane in the GESTIS substance database of the IFA , accessed on May 5, 2018(JavaScript required) .
- ↑ Data sheet 1,1,1-trichloroethane from Sigma-Aldrich , accessed on March 5, 2011 ( PDF ).
- ↑ Entry on 1,1,1-trichloroethane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 71-55-6 or 1,1,1-trichloroethane ), accessed on May 14, 2020.
- ↑ G. Myhre, D. Shindell et al .: Climate Change 2013: The Physical Science Basis . Working Group I contribution to the IPCC Fifth Assessment Report. Ed .: Intergovernmental Panel on Climate Change . 2013, Chapter 8: Anthropogenic and Natural Radiative Forcing, pp. 24-39; Table 8.SM.16 ( PDF ).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-22.
- ↑ a b c d Entry on trichloroethane. In: Römpp Online . Georg Thieme Verlag, accessed on July 23, 2015.
- ^ Franz von Bruchhausen: Hager's Handbook of Pharmaceutical Practice . Springer-Verlag, 1930, ISBN 978-3-540-52688-9 , pp. 1039 ( limited preview in Google Book search).
