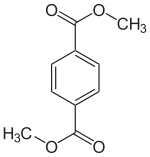
Dimethyl terephthalate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Dimethyl terephthalate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 10 O 4 | ||||||||||||||||||
| Brief description |
colorless, almost odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 194.19 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.36 g cm −3 |
||||||||||||||||||
| Melting point |
141 ° C |
||||||||||||||||||
| boiling point |
288 ° C |
||||||||||||||||||
| Vapor pressure |
<0.13 h Pa (30 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Dimethyl terephthalate (DMT) is an organic compound . It is the dimethyl ester of 1,4-benzene dicarboxylic acid ( terephthalic acid ).
Physical Properties
The flash point is 151 ° C, the ignition temperature is 520 ° C and the decomposition temperature is> 520 ° C. The explosion limits are between 0.8% (lower value) and 11.8% (upper value).
Manufacturing
Dimethyl terephthalate is mostly produced using the Katzschmann process. In this case, p - toluic acid methyl ester is selectively produced in a xylene isomer mixture by air oxidation, esterification and crystallization . After it has been separated off, this is oxidized again with air and esterified with methanol.
use
Dimethyl terephthalate is a basic material for the industrial production of polyesters , for example polyethylene terephthalate (PET) via the intermediate product bis (hydroxyethyl) terephthalate or polybutylene terephthalate (PBT). It is also used to manufacture polyester resins for foils, paints and adhesives and 1,4- dimethylolcyclohexane .
Individual evidence
- ↑ a b c d e f g Entry on dimethyl terephthalate in the GESTIS substance database of the IFA , accessed on December 20, 2019(JavaScript required) .
- ↑ a b c d Toxicological assessment of terephthalic acid dimethyl ester (PDF) at the professional association raw materials and chemical industry (BG RCI), accessed on August 22, 2012.
- ^ Bertram Philipp, Peter Stevens: Grundzüge der Industrielle Chemie , VCH Verlagsgesellschaft mbH, 1987, pp. 308–309, ISBN 3-527-25991-0 .
- ^ Klaus Weissermel , Hans-Jürgen Arpe : Industrial Organic Chemistry . 4th edition. Wiley-VCH, Weinheim 2003, ISBN 978-3-527-30578-0 , pp. 403 ( limited preview in Google Book search).
