Aldehydes
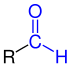
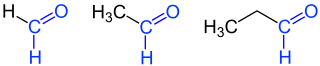
Aldehydes (from neo-Latin al coholus dehyd rogenatus, "dehydrated alcohol " or "alcohol from which hydrogen has been removed") are chemical compounds with the functional group -CHO, also known as the aldehyde group or the formyl group. In contrast to the ketones, the carbonyl group ( > C = O) of the aldehydes has a hydrogen and a carbon substituent. The simplest aldehyde methanal ( formaldehyde ), which has two hydrogen substituents, is an exception . Aldehydes with an alkyl radical ( i.e. alkane derivatives) are called alkanals ; their homologous series is nomenclaturally derived from the homologous series of alkanes. There are also multiple aldehydes - such as glyoxal , the simplest dialdehyde.
nomenclature
According to the IUPAC nomenclature, aldehydes are given the name of the alkane with the same number of carbon atoms with the suffix -al or -carbaldehyde. Accordingly, the aldehyde derived from methane is called methanal , the ethanal derived from ethane . If another functional group has a higher priority, the prefix “Formyl-” is used. If, on the other hand, the compound is a natural substance or a carboxylic acid, the prefix “Oxo-” is chosen.
The trivial name is derived from the Latin name for the carboxylic acid that is created when an oxygen atom is added . For methanal (H – CHO) this is methanoic acid (Latin acidum form icum, H – COOH), hence form aldehyde, for ethanal it is ethanoic acid (Latin acidum acet icum, CH 3 –COOH), hence acetaldehyde . The other trivial names are derived accordingly. Dicarboxylic acids in which a carboxylic acid group has been reduced to an aldehyde group are sometimes called semialdehydes .
Homologous series of the alkanals
| Number (carbon atoms) |
IUPAC name |
Common names | Molecular formula | Structural formula |
Boiling point in ° C |
|---|---|---|---|---|---|
| 1 | Methanal | formaldehyde | CH 2 O | −19.1 | |
| 2 | Ethanal | acetaldehyde | C 2 H 4 O | 20.1 | |
| 3 | Propanal | propionaldehyde propylaldehyde |
C 3 H 6 O | 48 | |
| 4th | Butanal | n -butyraldehyde | C 4 H 8 O | 74.8 | |
| 5 | Pentanal | Valeraldehyde amylaldehyde n -pentaldehyde |
C 5 H 10 O | 103 | |
| 6th | Hexanal | Capronaldehyde n -hexaldehyde |
C 6 H 12 O | 131 | |
| 7th | Heptanal | Enanthaldehyde heptyl n -Heptaldehyd |
C 7 H 14 O | 152.8 | |
| 8th | Octanal | Caprylaldehyde n- octylaldehyde |
C 8 H 16 O | 171 | |
| 9 | Nonanal | Pelargonaldehyde n -Nonylaldehyde |
C 9 H 18 O | 191 | |
| 10 | Decanal | Capric aldehyde n -decylaldehyde |
C 10 H 20 O | 208.5 | |
| 12 | Dodecanal | laurinaldehyde dodecyl |
C 12 H 24 O | 238 | |
| 14th | Tetradecanal | Myristyl aldehyde tetradecyl aldehyde |
C 14 H 28 O | 260 |
General empirical formula for the alkanals: C n H 2 n +1 CHO ( n = 0, 1, 2, 3, 4, ...)
There are also many other groups of aldehydes for which historical names are mostly used:
- Acrolein is derived from propene - an alkene .
- Benzaldehyde is derived from benzene , so it is an arylaldehyde.
- Furfural (furfural, furan-2-carbaldehyde) is derived from furan and is therefore a heteroarylaldehyde.
properties
Dipole-dipole forces arise between the aldehyde groups of alkanals , since the C = O double bond is very polar. Hydrogen bonds do not form because there is no oxygen-bonded hydrogen atom. That is why the boiling points of aldehydes are between those of alcohols and alkanes. Aldehydes can form hydrogen bonds with water because the oxygen atom has two free electron pairs and is negatively polarized. This is why short-chain aldehydes are readily soluble in water. In the case of longer-chain aldehydes, the effect of the non-polar alkyl radicals predominates, which makes the compounds insoluble in water. Many aldehydes have a characteristic odor.
Occurrence
Aldehydes are widely used as flavorings in foods such as wine . These often arise in fruit and vegetables from substances containing oleic , linoleic or linolenic acid during harvest, chopping or preparation. Hexanal can be found e.g. B. in apples, pears, peaches and cherries. ( E ) -2 -hexenal is found in apples, peaches, cherries and plums, the isomeric ( Z ) -2-hexenal in apples, pears, oranges and strawberries. ( Z ) -3-nonadienal occurs in cucumbers in addition to ( E, E ) -2,4-nonadienal, ( E, Z ) -2,6-nonadienal and ( Z, Z ) -3,6-nonadienal as an odor-giving flavor .
Above a certain concentration, however, such carbonyl compounds are often rated as rancid, fishy, metallic or as cardboard-like aromas and, overall, cause an old taste.
Manufacturing
The mild oxidation of primary alcohols in a non-aqueous medium produces aldehydes. They can be further oxidized to carboxylic acids.
- Ethanol reacts with copper oxide in a redox reaction to form acetaldehyde , copper and water .
The technically most important process for the production of aldehydes is the oxo synthesis , also known as hydroformylation . An alkene is reacted with a mixture of carbon monoxide and hydrogen in the presence of a suitable catalyst:

The hydroformylation of an alkene produces a mixture of n -aldehyde (middle) and i -aldehyde (right).
use
Formaldehyde (methanal) is produced in large quantities (21 million tons worldwide per year), more than any other aldehyde. It is used as a disinfectant, as a preservative for perishable goods such as cosmetics (formalin solution) and as a raw material in the chemical industry. The largest quantities were processed into aminoplasts and phenoplasts in the plastics industry until 1990 . In medicine, methanal in a 4–8% solution (formalin) is used as a fixative in histotechnology .
Aldehydes and ketones are also used in the manufacture of plastics , solvents , dyes , tannins , perfumes and medicines. Based on acrolein , DL methionine, a feed additive , is produced in quantities of more than 100,000 tons per year.
In medicine, formaldehyde and glutaraldehyde are used as surface and instrument disinfectants. Both aldehydes are effective against many different microorganisms . In particular, non-enveloped viruses and spore-forming bacteria (e.g. anthrax ), which are only accessible to a few disinfectants, can be reached in this way. Since aldehydes have an irritating effect on the skin and mucous membranes and occasionally cause allergies, these agents must be used carefully.
In the perfumery aldehydes since 1921 used ( Chanel No. 5 ).
Physiological importance
A number of aldehydes are found in the metabolism of cells. A special role is played by acetaldehyde (ethanal), which is produced in the course of the degradation of ethanol and is involved in the development of the so-called alcohol hangover .
proof
Aldehyde Spectroscopy
In the IR spectra of aldehydes and ketones one finds the intense characteristic band of the C = O stretching vibration in the range of 1690–1750 cm −1 .
In 13 C-NMR spectra the signal of the carbonyl carbon atom of aldehydes and ketones is found in a range of 195 and 210 ppm. The corresponding proton of the aldehyde group can be found in 1 H-NMR spectra as a sharp signal at around 10 ppm. This property makes identification by means of NMR spectroscopy particularly easy, since only a few protons have a resonance in this high range.
Reactions
Aldehydes are reactive compounds and can be easily oxidized to carboxylic acids .
- The C = O bond of the carbonyl group is strongly polar with the partial positive charge (δ +) on the carbon atom, which can be attacked by nucleophiles .
- Aldehydes with a hydrogen atom bonded to the α-carbon atom directly next to the carbonyl group can be in the keto or enol form - see also keto-enol tautomerism .
- In the case of aldehydes, it is observed that hydrogen atoms on the C atom adjacent to the carbonyl group are significantly more acidic than hydrogen atoms on "normal" C atoms. On the one hand, this is due to the fact that the carbonyl carbon is very poor in electrons and has an -I effect on neighboring bonds; on the other hand, after deprotonation, the negative charge on the oxygen of the carbonyl group can be delocalized ( -M effect ).
Nucleophilic addition
After attack by the nucleophile, the π- electron pair goes entirely to the oxygen , which is now negatively charged . In the protic solvent , this is compensated for by the uptake of protons, which creates an OH group instead of the carbonyl group.
Addition of water
Water + aldehyde ⇒ aldehyde hydrate ( geminal diol )
In aqueous solution, aldehydes are in equilibrium with the corresponding gem- diol, i.e. a hydrocarbon with two hydroxyl groups on one carbon atom. Usually the equilibrium is on the side of the aldehyde. In the case of trichloroacetaldehyde, however , the equilibrium is on the side of the geminal diol.
Addition of alcohols
Alcohol + aldehyde ⇒ hemiacetal
Hemiacetal + alcohol ⇒ acetal + water
Example: ring closure of grape sugar (glucose)
See also: acetal formation
Addition of nitrogen nucleophiles
Prim. Amine + aldehyde ⇒ imine (Schiff base) + water
Sec. Amine + aldehyde ⇒ enamine + water
Oxidation to carboxylic acid (important for proof)
Aldol reaction
The CH-acidic H atom in the α position can be split off by bases . The resulting enolate anion adds to the carbonyl carbon of another aldehyde molecule. An aldol is formed , an addition product of alcohol (OH group) and aldehyde. In this way, CC bonds can be made. If the aldol formed is then dehydrated, it is called aldol condensation , which results in α, β-unsaturated aldehydes.
Mixed aldol reaction
Mixed aldol reactions cannot usually be carried out in a one-pot reaction, since four possible products can and do form. An exception is when one of the two aldehydes cannot be enolized, i.e. does not have a CH-acidic hydrogen atom. In this case only a mixed aldol is possible. An example of non-enolizable aldehydes are aromatic aldehydes (see: Benzaldehyde ). In this way, cinnamaldehyde , an important fragrance , is obtained in a Knoevenagel condensation .
Pinacol coupling
Substituting aldehydes with an alkali metal (eg sodium ) in a way, forms a radical - anion , the dimerized quickly. The hydrolysis produces a pinacol (traditional name for a 1,2- diol , i.e. a diol with vicinal hydroxyl groups ). Starting from an α, ω-dialdehyde, cyclic 1,2-diols are obtained analogously by an intramolecular reaction.
Individual evidence
- ↑ Duden, German Universal Dictionary, 4th ed., Mannheim, 2001.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-1 - 3-523.
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/31291#section=Experimental-Properties
- ^ Siegfried Hauptmann : Organic chemistry. 2nd reviewed edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 565, ISBN 3-342-00280-8 .
- ↑ a b Wolfgang Legrum: Fragrances, between stench and fragrance. Vieweg + Teubner Verlag (2011) pp. 82-85, ISBN 978-3-8348-1245-2 .
- ↑ Werner Köhler, Rainer Ansorg: Medical Microbiology. Elsevier, Urban & Fischer Verlag, 2001, ISBN 3-437-41640-5 , p. 92.
- ↑ KPC Vollhardt, NE Schore: Organic Chemistry . Ed .: H. Butenschön. 4th edition. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2005, ISBN 3-527-31380-X , p. 862-863 .