formaldehyde
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | formaldehyde | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | CH 2 O | |||||||||||||||||||||
| Brief description |
colorless, pungent penetrating smelling gas |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| characteristics | ||||||||||||||||||||||
| Molar mass | 30.03 g mol −1 | |||||||||||||||||||||
| Physical state |
gaseous |
|||||||||||||||||||||
| density |
0.815 g cm −3 (−20 ° C) |
|||||||||||||||||||||
| Melting point |
−117 ° C |
|||||||||||||||||||||
| boiling point |
−19 ° C |
|||||||||||||||||||||
| Vapor pressure |
0.43-0.44 M Pa (20 ° C) |
|||||||||||||||||||||
| solubility |
easily soluble in water |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
DFG / Switzerland: 0.3 ml m −3 or 0.37 mg m −3 |
|||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| Thermodynamic properties | ||||||||||||||||||||||
| ΔH f 0 |
−108.6 kJ / mol |
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Formaldehyde ( ˈfɔɐ̯m.aldehyːt , also … ˈhyːt , systematic name methanal ) is an organic-chemical compound with the empirical formula CH 2 O and the simplest member of the aldehyde group . Under standard conditions , formaldehyde is a gas with a pungent odor.
With around 21 million tons of annual production (as of 2019, based on 100% formaldehyde), formaldehyde is one of the most commonly produced organic chemicals. The technical production of formaldehyde takes place catalytically through the oxidation or dehydrogenation of methanol , for example in the silver catalyst process or the Formox process . In the chemical industry, it is used in particular as a raw material in the production of phenolic and urea resins . Another polymer is paraformaldehyde , which is used in cell biology, among other things. It is a powerful antiseptic and disinfectant that is available as a 40 percent solution of the aldehyde in water and is used as a fungicide and preservative .
In nature, formaldehyde occurs as an oxidation product of terpenes and as a metabolic product of bacteria , which aerobically metabolize substrates with a carbon atom such as methanol , methane or methylamine to carbon dioxide . Formaldehyde is part of the human metabolism. It is classified as a carcinogen.
nomenclature
The systematic IUPAC name methanal for molecular formaldehyde is derived from methane by adding the suffix -al for aldehydes. The preferred IUPAC name formaldehyde comes from " formica ", the Latin word for the ant , since formaldehyde can be converted into formic acid through oxidation .
The aqueous solution of formaldehyde is called formalin or, less commonly, formol . It was marketed as “Formalin” by Schering and as “Formol” by Hoechst from 1893 onwards . A saturated aqueous solution contains around 40% by volume of formaldehyde or 37% by mass and is referred to as "100% formalin". A stabilizer such as methanol is often added to this in order to suppress the polymerization . A typical commercial formalin can contain up to 12% methanol. Production figures for formaldehyde are usually given on the basis of the 37% mass fraction of formalin solution.
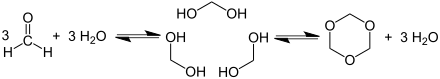
In aqueous solution, formaldehyde is in its hydrated form as methanediol with the formula CH 2 (OH) 2 . Depending on the concentration and temperature, this compound is in equilibrium with various oligomers called paraformaldehyde with a typical degree of polymerization of 8 to 100 units. Heating reverses the reaction and releases formaldehyde again from paraformaldehyde. Under the trade name Formcel from Celanese are solutions of formaldehyde in methanol (Methyl Formcel) with 55.0% mass fraction formaldehyde, 34.5% mass fraction methanol and 10.5% mass fraction water as well as solutions in butanol and isobutanol (Butyl Formcel) with 40% Mass fraction of formaldehyde, 53% mass fraction of butanol and 7% mass fraction of water available. Trioxane is a trimer of molecular formaldehyde.
story
Alexander Michailowitsch Butlerow synthesized formaldehyde or paraformaldehyde in 1855 by converting diiodomethane with silver acetate . He saponified the acetate initially formed by boiling it with water and concentrated the resulting solution in vacuo. Butlerow, who called the substance "dioxymethylene", did not realize that he had produced paraformaldehyde. He investigated the chemistry of formaldehyde further and in 1861 discovered the formose reaction , in which a mixture of sugars is formed from formaldehyde.
Development of technical synthesis
In search of the first member of the aldehyde series, August Wilhelm von Hofmann carried out the first targeted representation in 1867 by the dehydrogenation of methanol on a glowing platinum wire . This laboratory process allowed the production of a few liters of formaldehyde solution from methanol and thus further studies on the chemistry of this aldehyde. In 1872 Adolf von Baeyer discovered its condensation with phenol to form phenol-formaldehyde resins , but without pursuing the discovery any further.
Bernhard Tollens optimized the yield by regulating the ratio of methanol to air; To avoid explosions, he developed a flashback arrestor in the form of an asbestos wad , which he inserted between the methanol reservoir and the platinum spiral . Oskar Loew improved the formaldehyde synthesis by using initially iron (III) oxide and later copper as a catalyst.
In 1888, the Mercklin & Lösekann company started the commercial production of formaldehyde in Seelze . From 1889, the need for formaldehyde for dye production increased . So could acridine by the reaction of diphenylamine with formaldehyde, catalyzed zinc chloride are prepared. Acridine is the base for acridine dyes such as acridine orange and acridine yellow , which until then was only obtained from coal tar .
The company Meister, Lucius and Brüning , which took over a patent for the production of formaldehyde from Jean Joseph Auguste Trillat in 1890 , had considerable interest in the development of medical applications for aqueous formaldehyde solutions. In 1892 they commissioned the Frankfurt doctor Ferdinand Blum to investigate the antiseptic properties of formaldehyde. Blum demonstrated the bacteriocidal properties of a 4 percent formaldehyde solution on bacteria such as Bacillus anthracis and Staphylococcus aureus . By chance, during his experiments, he discovered the possibility of fixing tissue samples with formaldehyde.
Plastics made from formaldehyde
Formaldehyde found its first major technical use through the invention of Galalith , a thermosetting plastic based on casein and formaldehyde, patented by Adolf Spitteler and Wilhelm Krische in 1897 . The plastic was successfully marketed and was used for hair combs and accessories , knitting needles, pens, umbrella handles, white piano keys, electrical appliances and much more. In the German Reich in 1913 about 6% of the total milk production was used for the production of Galalith.
Baeyer's work on the condensation of phenol and formaldehyde was taken up by various chemists, for example by Arthur Smith in 1899, by A. Luft in 1902, by F. Hensche in 1903, who investigated an alkaline-catalyzed condensation, and in 1905 by H. Story. But only Leo Baekeland recognized the potential of this synthesis in 1907 with the production of Bakelite , the first fully synthetic plastic. His company, General Bakelit, started the technical production of Bakelite in 1910.
Bakelite products, however, tended to darken and in 1918, while looking for clearer plastics, chemist Hans John discovered urea resins. The demand for urea and phenolic resins increased the need for formaldehyde significantly.
Large-scale production
It was not until Matthias Pier and Alwin Mittasch developed the production of methanol from synthesis gas in the high pressure process on zinc oxide - chromium oxide catalysts by Matthias Pier and Alwin Mittasch in the 1920s that the development of large-scale production was initiated. Up to this point in time, the commercial production of methanol was only a by-product of charcoal production , which was started in Germany in 1857 by Dietze, Morano & Cie. began in Lorch , where in addition to charcoal and methanol, acetic acid and methyl acetate were obtained as further products .
In the 1930s, Homer Burton Adkins and Wesley R. Peterson developed the Adkins-Peterson reaction for the direct oxidation of methanol to formaldehyde. Adkins, who was working at Bakelite Corporation at the time, used an iron-molybdenum oxide catalyst to do this. The annual production was around 25,000 tons in 1931 and had quadrupled to around 100,000 tons by 1943.
Use in the wood industry
In the 1940s, a factory in Bremen produced the first chipboard using urea-formaldehyde resins, thus generating high demand in the construction and furniture industries. The processing of wood chips made possible by this increased the degree of utilization of trees from 40% to 80%.
Occurrence
Biological occurrence
In nature, for example, formaldehyde occurs as an intermediate product in mammalian cells during normal metabolism . In this way, about 878 to 1310 milligrams per kilogram of body weight are formed per day in humans . For a person weighing 70 kilograms, this corresponds to 61 to 92 grams of formaldehyde per day. The half-life is 1 to 1.5 minutes. Humans exhale around 0.001 to 0.01 mg / m 3 of formaldehyde, with no significant difference between smokers and non-smokers. The formaldehyde level in the blood varies between 0.4 and 0.6 μg cm −3 and in the urine between 2.5 and 4.0 μg cm −3 . The daily intake is up to about 14 mg. Formaldehyde is also found in wood and diffuses outside in small amounts.
Methylotrophic bacteria like Methylophilaceae or methanotrophic bacteria like Methylococcaceae metabolize a number of compounds with only one carbon atom such as methanol, methane , methylamine and dichloromethane as an energy source. These compounds are metabolized via the cytotoxin formaldehyde. The oxidation of formaldehyde to carbon dioxide is an important part of the metabolism of these aerobic bacteria.
Food and luxury foods
Formaldehyde occurs naturally in fruits such as apples and grapes . The lowest formaldehyde concentration in food could be measured in fresh milk, with a content of 0.013 to almost 1 mg / kg. The highest level was measured in frozen hake at 232–293 mg / kg.
When consuming one pack of cigarettes, the smoker absorbs around 3 mg of formaldehyde per day. Some e-cigarettes contain substances such as propylene glycol, which can release formaldehyde when vaporizing. The e-cigarette smoker consumes around 14 mg of formaldehyde per day with the same consumption.
In conservation processes such as smoking , formaldehyde is released through the pyrolysis of hardwoods . It has a microbiocidal effect against yeasts and molds and cross-linking proteins .
| food | Formaldehyde in [mg / kg] |
|---|---|
| Meat and poultry | 5.7-20 |
| fish | 6.4-293 |
| milk and milkproducts | 0.01-0.80 |
| Sugar and sweeteners | 0.75 |
| fruit and vegetables | 6-35 |
| coffee | 3.4-16 |
| Alcoholic drinks | 0.27-3.0 |
Atmospheric occurrences
Formaldehyde is a ubiquitous trace chemical and the most abundant carbonyl compound in the atmosphere. It arises from the photochemical reaction of hydrocarbons or the incomplete combustion of fossil fuels and biomass . The combustion of fuel and wood are the predominant sources of anthropogenic atmospheric formaldehyde, with the major emissions coming from biogenic sources such as the oxidation of methane and isoprene .
The photolysis of formaldehyde may play a role in air pollution in urban environments. Two reaction pathways are assumed for the photolytic decay, one of which proceeds via the formation of hydrogen and carbon monoxide.
The second pathway leads to the formation of hydrogen and formyl radicals.
The importance of this reaction path arises from the fact that these radicals play an important role in the oxidation of nitric oxide to nitrogen dioxide and the formation of ozone . Atmospheric sinks for formaldehyde are reactions with hydroxyl radicals and photolysis . In the tropical atmosphere, the mixing ratio of formaldehyde is around 1 ppb, one of the main sources here is the oxidation of methane. An important natural source of emissions is the atmospheric oxidation of methane. It is believed that photochemical processes in the primordial atmosphere led to the formation of around 3 million tons of formaldehyde per year. The precipitation of formaldehyde and subsequent reactions of formaldehyde in primeval waters possibly led to an abiotic synthesis of complex organic molecules and thus possibly enabled the origin of life.
Extraterrestrial occurrences
Radio astronomers detected formaldehyde as the first polyatomic organic molecule in the interstellar medium in many regions of our galaxy by means of the ground state rotation transition at 4830 MHz, mainly in the vicinity of young, massive star objects. Studying the emissions of formaldehyde is useful in deriving the spatial density and kinetic temperature of the dense gas in the Milky Way and other galaxies, such as NGC 660 .
According to radio-astronomical measurements of the ground state rotational transition of formaldehyde, the ratio of 12 C to 13 C in the galactic disk is between 5 and 8 kiloparsecs about 50. This is less by a factor of 2 compared to the local interstellar medium and qualitatively agrees with predictions from galactic evolution models that predict higher metallicity of the gas in the inner galactic disk.
It is believed that formaldehyde is an important precursor for a large number of more complex organic molecules such as amino acids in the interstellar medium. Using a mass spectrometer on board the Rosetta probe , formaldehyde was detected in the tail of the Churyumov-Gerasimenko comet . Using the Atacama Large Millimeter / submillimeter Array , the distribution of formaldehyde in the coma of comets C / 2012 F6 (Lemmon) and C / 2012 S1 (ISON) was measured and described in detail. Multi-dimensional solid-state NMR spectroscopy has identified functional groups in insoluble organic matter in carbon-containing chondrites , which are possibly polymerization products of formaldehyde. Extraterrestrial formaldehyde is being discussed as a possible source of organic compounds that led to life on earth.
Manufacturing
The large-scale production of formaldehyde now takes place almost exclusively using two established process principles, both of which rely on the starting material methanol and have been modified differently depending on the manufacturer: oxidative dehydrogenation and oxidation of methanol.
In the 1970s and 1980s , formaldehyde was produced in the USA by the radical oxidation of propane and butane (C3 / C4 cut). For some time, formaldehyde was also produced in Japan through the oxidation of dimethyl ether . Due to the unsatisfactory selectivity and high production costs, these two processes could not prove themselves in the long term and are no longer carried out today. The global annual production of formaldehyde was around 21 million tons in 2019 (based on 100% formaldehyde). The largest production regions were the Asia-Pacific region , followed by the European Union and the United States . The production of formaldehyde usually takes place near the industrial consumer, as stability-related problems can arise during long-distance transport. The product usually reaches the consumers via a pipeline network. World trade in formaldehyde is low compared to the volume of production. The larger manufacturers in 2017 included Dynea Chemicals , Perstorp , Georgia-Pacific , Celanese , Ercros , BASF and many others.
Oxidation of methanol (Formox process)
The first process principle is based on a simple oxidation reaction. Methanol is then air oxygen at temperatures of 350-450 ° C without pressure on iron (III) oxide - and molybdenum (VI) oxide - catalysts in tube bundle reactors converted to formaldehyde.
The conversion takes place in the gas phase and with a large excess of atmospheric oxygen. The considerable heat of reaction (ΔH R = −159 kJ mol −1 ) is dissipated with the help of coolants such as molten salt , pressurized water or oils, which flow around the pipes, and used to generate superheated high-pressure steam . The catalyst is arranged as a fixed bed in the reactor . The catalytically active compound is iron (III) molybdate [Fe 2 (MoO 4 ) 3 ], which is formed from the catalyst precursor during the reaction. This acts as an oxygen carrier and oxidizes the resulting hydrogen to water . The reduced catalyst is regenerated simultaneously with atmospheric oxygen. A catalyst life of around two years is thus achieved. The only major side reaction that occurs is the complete oxidation (combustion) of formaldehyde to carbon dioxide and water. The methanol conversion is about 95-99% and the selectivity to formaldehyde reaches 91-94%. The dominant method nowadays of methanol oxidation was from Perstorp developed and Reichhold and is used as Formox process (from form aldehyde by ox indicated idation).
With the Formox process, urea-formaldehyde concentrates can be easily produced, but the aqueous formaldehyde solution in the silver process is of higher quality due to its lower formic acid content.
Oxidative dehydrogenation of methanol
The second process relates to an oxidative dehydrogenation of methanol, also known as the silver or silver catalyst process . In the first step, methanol is dehydrogenated to formaldehyde over metallic silver catalysts at temperatures of 600–720 ° C.
The silver catalyst is arranged in the reactor as a fixed bed , mostly as crystals , nets or impregnated on silicon carbide . The dehydrogenation reaction is an endothermic reaction (ΔH R = +84 kJ · mol −1 ) and is favored by increased temperature.
In a secondary step, the hydrogen produced is burned with air oxygen in an exothermic reaction (ΔH R = –243 kJ · mol −1 ) to form water.
The oxidation is controlled by the amount of oxygen added in order to achieve an adiabatic mode of operation. The life of the catalyst is 2-4 months. As a result, the catalyst has to be changed significantly more often than with the Formox process . On the other hand, the silver catalyst can be electrolytically regenerated very easily and without material loss.
Due to the rapid thermal decomposition and the further oxidation of the formaldehyde to formic acid , extremely short residence times (less than 0.01 s) must be observed. For this reason, mesh catalysts are preferred which allow a short contact time on the thin catalyst layer and rapid cooling in 0.1-0.3 s to about 150 ° C. Furthermore, due to the low pressure loss when using such catalysts, very high flow speeds can be achieved, which ensures efficient heat dissipation.
There are process variants according to BASF , Bayer , Borden, Celanese , Degussa , DuPont , ICI and Mitsubishi , which differ in the type of catalyst, the reaction temperature and the processing of the formaldehyde.
The three common types of the silver catalyst process used are the BASF process with water injection and almost complete conversion, the incomplete conversion with subsequent distillation according to the ICI process and the gas recycling process used primarily in China.
According to the BASF process variant , methanol and water are mixed with air and fed into the reactor (2) via the evaporator (1) . The silver catalyst is arranged in this as a fixed bed (e.g. nets, crystals) and the temperature is kept at around 680-720 ° C. After the reaction, the hot reaction gases in the gas cooler (3) are quickly cooled to 150 ° C. This is done indirectly via a heat exchanger system that is connected to both the evaporator and the reactor. The cooling takes place with water. Optimal reaction management makes it possible to generate around 70 kg of water vapor per ton of formaldehyde , which can be used internally in the plant or in the plant network . The cooled reaction gases are then passed into two absorption columns (4) and (5) in which formaldehyde is washed out in countercurrent with water or with the circulating formaldehyde solution. This gives a 44% aqueous formaldehyde solution. The exhaust gases from the second absorption column are either burned directly (energy generation) or partially returned to the evaporator.
The yield in this process is between 86.5 and 90.5 mol%. The aqueous formaldehyde solution still has a content of 1-2% by weight of methanol and 0.01% by weight of formic acid, which, however, is not a quality problem and is generally handled as a salable product.
Oxidation of steam cracker products
Formaldehyde can be obtained by oxidation of steam cracker products. The C3 cut, which contains molecules with three carbon atoms such as propane and propene , and the C4 cut, which contains molecules with four carbon atoms such as butane , butene and butadiene , are oxidized. The oxidation of the C3 / C4 cut can be carried out with or without a catalyst. Formaldehyde is produced alongside other oxygen-containing components such as methanol, acetaldehyde , acetic acid and acetone . In order to avoid explosions, this process must either be carried out with a large excess of air or with an excess of hydrocarbons. Another suitable diluent is steam. To avoid secondary reactions, the reaction mixture must be quickly cooled to below a temperature of about 150 ° C. This is done by quenching with injected water.
Overall , the reactions are exothermic - the correspondingly hot gas mixture has to be cooled down quickly to avoid side reactions. The formaldehyde gas produced is then extracted in gas scrubbers using water or a urea solution , an aqueous formaldehyde solution or a urea-formaldehyde concentrate being produced . In addition to unreacted methanol, the resulting solutions also contain small amounts (about 100–300 ppm) of formic acid (HCOOH).
characteristics
Physical Properties
Formaldehyde is a colorless, pungent smelling substance that is gaseous at room temperature . As a gas, its odor is still perceptible in concentrations of 0.05–1 ml / m 3 . It boils at −19 ° C. The density of liquid formaldehyde is 0.815 grams per cubic centimeter (g · cm −3 ) at −20 ° C. The melting point is −118 ° C.
Liquid and gaseous formaldehyde polymerizes easily up to a temperature of 80 ° C, at higher temperatures it is monomeric. The rate of polymerization depends on many factors such as pressure or humidity and is catalyzed by traces of acid. Formaldehyde is flammable and ignites from a temperature of 430 ° C. It forms explosive mixtures with air in a wide range of concentrations. The explosion range is between 7% by volume (87 g / m 3 ) as the lower explosion limit (LEL) and 73% by volume (910 g / m 3 ) as the upper explosion limit (UEL).
The dipole moment of formaldehyde is 2.330 Debye (D), the energy of formation −104.7 kilojoules per mole (kJ · mol −1 ). The critical temperature is 134.85 ° C, the critical pressure 65.9 bar .
The crystal structure of formaldehyde was determined at a temperature of 15 Kelvin by neutron diffractometry . Formaldehyde crystallizes in the tetragonal crystal system with the space group P 4 2 1 c with eight molecules per unit cell. The molecules are arranged in four-membered squares with strong CO bonds that connect the members of a square.
Molecular Properties
The electron density in the oxygen of the formaldehyde is in the occupied π orbital, the HOMO , greatly increased compared to the carbon. On the other hand, the orbitals in the unoccupied π * orbital, the LUMO, are larger at carbon, so formaldehyde is a good electrophile . In reactions of formaldehyde with strong nucleophiles such as thiols , amines or amides , acid catalysis is often not required. The hydroxymethyl derivatives formed typically react further. In the presence of acids, it reacts in electrophilic aromatic substitution reactions with aromatic compounds which lead to hydroxymethylated derivatives.
Formaldehyde is a planar molecule with an axis of rotation and two mutually perpendicular mirror planes and is called C 2v -symmetrical according to Arthur Moritz Schoenflies . The C = O bond length is 120 picometers , the CH bond length is 110 picometers. The HCH angle is 116.16 °, the HCO angle accordingly 121.92 °.
The wave number of the CH stretching vibration is 2782, that of the C = O stretching vibration is 1746 cm −1 . The wave number of the CH 2 bending vibration is 1500 cm −1 .
Chemical properties
Formaldehyde reacts with itself and with other reactants in a number of syntheses to form a wide variety of products. Among these reactions are oxidation-reduction reactions, addition or condensation reactions with organic and inorganic substances, and self-polymerization reactions.
Oxidation-reduction reactions
In the presence of bases, formaldehyde disproportionates to formate and methanol in the Cannizzaro reaction .
With aldehydes without hydrogen in the α-position to the carbonyl group, formaldehyde reacts in a cross Cannizzaro reaction to form alcohol and formate.
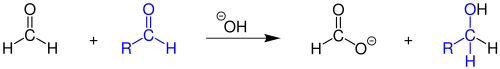
In the Tollens sample , formaldehyde reacts with the soluble diammine silver (I) complex ([Ag (NH 3 ) 2 ] + ) in alkaline solution to form formic acid, silver and ammonia. The reaction is a general detection reaction for aldehydes.
Addition and condensation reactions
As the simplest aldehyde, formaldehyde has a special position in its chemical behavior, since the aldehyde group is only bound to hydrogen. Some of the typical aldehyde reactions proceed normally, such as the synthesis of cyanohydrin to glycolonitrile .
With ammonia, on the other hand, no imine is formed , but hexamethylenetetramine .
Formaldehyde is very soluble in ethanol , diethyl ether and water. In aqueous solution, an aldehyde hydrate (methanediol) is formed, whereby the equilibrium of this reaction - unlike z. B. Ethanal - is almost 100% on the hydrate side. The hydrate reacts slightly acidic (pK s 13.3).
Formaldehyde enters into a number of other condensation reactions with a wide range of reactants, such as in sulfomethylation or the Mannich reaction . The Mannich reaction is an aminoalkylation of a CH-acidic compound with formaldehyde and a primary or secondary amine or ammonia . The product is a β-amino carbonyl compound known as Mannich base.
Nitromethane reacts in a Henry reaction in excess formaldehyde to form 2-nitro-1,3-dihydroxy-2-hydroxymethyl propane . Basic compounds such as amines catalyze the reaction.
Formaldehyde reacts with benzene and hydrogen chloride in the Blanc reaction catalyzed by zinc chloride or other Lewis acids to form chloromethylarenes.
Formaldehyde reacts with cobalt carbonyl hydride in a reaction similar to hydroformylation .
By inserting carbon monoxide into the cobalt-carbon bond , an acyl complex is formed, which reacts with another equivalent of cobalt carbonyl hydride to form glycolaldehyde and dicobalt octacarbonyl .
In the 1960s, the reaction of formaldehyde, carbon monoxide, water, and sulfuric acid produced glycolic acid. Esterification with methanol and subsequent hydrogenation yielded ethylene glycol . Annual production using this process was around 60,000 tonnes per year in the mid-1960s, but it was discontinued in 1968 for cost reasons.
In crossed aldol reactions , formaldehyde reacts as an enolate anion acceptor. Formaldehyde reacts with acetone to form 4-hydroxy-2-butanone . Formaldehyde reacts with Grignard compounds to alcohols after hydrolysis.
Formaldehyde reacts with one or two equivalents of alcohol to form half or full acetals .
Self-polymerization reactions
In the formose reaction, sugars are formed through the self-condensation of formaldehyde. Bases of divalent metals such as calcium hydroxide or barium hydroxide catalyze this reaction. Ronald Breslow proposed a catalytic cycle in 1959. The reaction sequence includes aldol reactions , retro-aldol reactions and Lobry-de-Bruyn-Alberda-van-Ekenstein rearrangements with the formation of intermediates such as glycolaldehyde , glyceraldehyde , dihydroxyacetone and tetroses . The term formose is a trunk word made up of form aldehyde and aldose.
Formaldehyde easily polymerizes in the presence of traces of acid to give polyoxymethylenes or it trimerizes to give trioxane . The reaction is reversible, at higher temperatures the polymers and oligomers break down again into formaldehyde.
use

Formaldehyde is one of the most important organic raw materials in the chemical industry and is used as a raw material for many other chemical compounds. By far the largest market is in the area of urea-formaldehyde resins, phenoplasts, polyoxymethylene and a number of other chemical intermediates such as pentaerythritol . Formaldehyde is used, among other things, in the production of dyes , pharmaceuticals and in textile finishing . Since formaldehyde, like all aldehydes, is a strong reducing agent , it is used to kill germs. Formaldehyde is used in the laboratory for the Mannich reaction and the Blanc reaction , among other things .
Polymer manufacturing
Aminoplasts
With urea formaldehyde reacts to urea-formaldehyde resins (UF-resins U REA F , with ormaldehyden) melamine to melamine-formaldehyde resins (MF-resins), both to the aminoplasts include. In the first step, monomethylolurea and dimethylolurea are produced:
Further condensation gives rise to chain-like polymers which can optionally be crosslinked. Resins based on urea-formaldehyde are the most important types of adhesive resins for the manufacture of wood-based materials such as chipboard, fiberboard and hardwood plywood. The lack of water resistance of the cured resin due to the reversibility of the aminomethylene bond can be remedied by adding substances such as melamine .
By far the largest area of application for formaldehyde is the production of urea-formaldehyde resins, which are used as binders for non-structural wood-based materials such as chipboard and medium-density fibreboard (MDF).
Melamine-formaldehyde resins are used as impregnating resins when there are increased demands on moisture resistance, for example for applying decorative papers to laminate floors. MF resins are used in the automotive industry in the form of clear coats.
N-methylol compounds made from formaldehyde and urea, such as methylolurea, which form aminoplasts in the fibers through further condensation, are used as textile auxiliaries in cellulose fibers such as cotton fibers or viscose fibers . These serve to improve the creasing and shrinking behavior and thus increase the dimensional stability of textiles. The polycondensation of the N-methylol compounds usually takes place in an acidic medium at an elevated temperature. A certain amount of formaldehyde is produced during condensation. The amount of aminoplasts stored is around 8% based on the weight of the textile. From a health point of view, a small amount of free and releasable formaldehyde must be ensured during textile finishing. Textiles that come into contact with the skin when used as intended and that contain more than 0.15 percent free formaldehyde must be labeled accordingly.
Phenoplasts
Phenol-formaldehyde resins (PF) or phenoplasts are synthetic polymers made by the condensation reaction of phenol or substituted phenol with formaldehyde. Novolaks or resols are formed, depending on whether the condensation is acidic or basic . Novolaks are low molecular weight polymers that are produced by the acid-catalyzed condensation of formaldehyde with a mixture of cresols . Novolaks are used as photoresist materials in microelectronics.
Resoles are products of the base-catalyzed phenol-formaldehyde condensation. They are made with an excess of formaldehyde to phenol. The reactive species are phenates , which are formed by deprotonation of phenol. As thermosets , the hydroxymethylphenols formed cross-link when heated to about 120 ° C, forming methylene and methyl ether bridges with the elimination of water. A high degree of crosslinking across the levels of Resitol and Resit gives the Resoles hardness, thermal stability and chemical resistance.
Phenol-formaldehyde resins are added to hexamethylenetetramine as a curing component. It is produced industrially by the reaction of six equivalents of formaldehyde with four equivalents of ammonia.
The co-condensation of phenol, phenolsulphonic acid and formaldehyde creates cation exchangers . These network polymers have firmly bound, anionic sulfate groups as well as freely moving cations.
Polyoxymethylene
Polyoxymethylene is a thermoplastic used in precision parts that require low friction and high dimensional stability. Polyoxymethylene is characterized by high strength , hardness and rigidity . Due to its high crystallinity , it is uncolored, opaque white. The automotive and electronics industries use injection molded POM for technical components such as gears, ball bearings and fasteners.
Pentaerythritol manufacture
Pentaerythritol is produced via a base-catalyzed polyaddition reaction between acetaldehyde and three equivalents of formaldehyde. The intermediate reacts in a cross Cannizzaro reaction with a fourth equivalent of formaldehyde to form pentaerythritol.
It is mainly used to make polyfunctional compounds and can be found in plastics, paints, cosmetics and many other applications. It is also used to manufacture explosives such as nitropenta and pentaerythritol trinitrate .
Methylenediphenyl isocyanate
The first step in the production of methylenediphenyl isocyanates (MDI) is the reaction of aniline and formaldehyde using hydrochloric acid as a catalyst.
A mixture of diamine precursors and the corresponding polyamines is produced. The global production of methylenediphenyl isocyanates in 2018 was around 9.8 million tons, for which around 1.2 million tons of formaldehyde were required. The manufacture of methylenediphenyl isocyanates is a rapidly growing market for formaldehyde. The main applications are polyurethane foams , paints , adhesives , elastomers and sealants, which are used in construction , household appliances, shoes and other consumer goods, as well as in the automotive industry.
1,4-butanediol
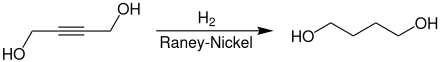
The industrial synthesis of 1,4-butanediol takes place via the reaction of acetylene with two equivalents of formaldehyde.
The 2-butyne-1,4-diol formed in the first step gives 1,4-butanediol through hydrogenation.
1,4-Butanediol is used as a solvent and in the manufacture of plastics, elastic fibers and polyurethanes. At a higher temperature in the presence of phosphoric acid, it cyclizes with elimination of water to tetrahydrofuran , an important secondary product.
Formaldehyde releasers
Formaldehyde is mostly used in cosmetic products in the form of a formaldehyde releaser . These are condensation products of formaldehyde such as diazolidinyl urea , which slowly release this in cosmetic products as a biocide for preservation. The EU regulation 2019/831 amending the Cosmetics Regulation provides for formaldehyde to be removed from the list of permitted substances and to be included in the list of substances that are prohibited in cosmetic products.
Appendix V of the EU Cosmetics Regulation regulates the use of formaldehyde in cosmetic products in the European Union. It is approved as an active ingredient in nail hardeners up to a concentration of 5% and as a preservative in oral care products up to 0.1% and generally in cosmetics up to 0.2%. If a concentration of free formaldehyde in the cosmetic product of 0.05% is exceeded, this must be declared in the form of the statement "Contains formaldehyde".
Dead vaccines
Formaldehyde is used in vaccine production to inactivate vaccine viruses (e.g. poliovirus ) or bacterial toxins (e.g. diphtheria toxin , tetanus toxin or pertussis toxin ). After purification, the finished vaccine preparation may contain a maximum of 200 mg (human vaccines) or 500 mg (animal vaccines) formaldehyde per liter. With human vaccines this corresponds to a maximum concentration of max. 0.2 mg / ml or 0.02%. Typically 1–200 µg per vaccine is injected. The amount of a single vaccination in humans is approximately at least 600 times less than the amount that can cause toxicity in animal studies. Since the amount of formaldehyde is often below the maximum allowed anyway and a concentration of 1% is usually used for a patch test for formaldehyde for allergy testing , the amount of formaldehyde in any vaccine cannot trigger any skin reactions - even if it is directly in or would be applied to the skin. About 10 times more formaldehyde circulates in the blood than is contained in a vaccination. The amount of formaldehyde contained is so small that the physiological formaldehyde content of the muscle is even diluted by vaccination. There is therefore no risk of formaldehyde after vaccination.
Preservation of anatomical and biological specimens
4 to 8 percent formaldehyde solution is used as a common fixative in histotechnology . Formaldehyde is a protein crosslinking additive fixant, stops the autolysis and putrefaction of tissue samples and makes them permanently durable. The rule of thumb is a penetration speed of 1 mm / h. The rate of crosslinking is considerably slower than the primary accumulation of formaldehyde; at least 2-3 days are required for adequate fixation. Methylene bridges and bridges over Schiff's bases are formed. The connection can be reversed by washing out in water or by the action of hot buffer solutions of different pH values (antigen retrieval). Methylene bridges should be stable. The crosslinking and modification of biomolecules with formaldehyde can be reversed by heating and / or by adding bases .
Such a formaldehyde solution is also used for the preservation of corpses and for the preservation of anatomical and biological specimens such as insects, first proposed by Isaak Blum in 1893 . Since material inserted in this way is durable for years, it can easily be used as illustrative or comparative material in medicine and biology for research and teaching purposes. The British artist Damien Hirst preserved a shark as a work of art in formaldehyde for artistic purposes .
Despite the health risks posed by formaldehyde, it is still largely indispensable in the preservation and preservation of tissues, particularly due to its general antiseptic properties. However, the technical conversion of the work areas to comply with the occupational exposure limit, for example by means of suction directly at the work area and reducing the concentration of formaldehyde in preservation solutions, is a central issue in modern anatomy and pathology .
Disinfection and sterilization
Formaldehyde is used in a variety of ways for disinfection and sterilization . For room disinfection, formaldehyde is applied in gaseous form or in an aqueous solution to all surfaces in a room. In addition to evaporation, formaldehyde can be atomized or substances can be used that release formaldehyde. Furthermore, disinfection can be carried out by wiping with agents containing formaldehyde. Formaldehyde is adsorbed by surfaces and has to be thoroughly removed by rinsing after treatment. For small medical parts, the fumigation with formaldehyde can take place in formaldehyde sterilizers.
In intensive animal husbandry , formaldehyde is used as a fumigant to prevent infectious diseases caused by viruses or bacteria. In chicken rearing and fattening, for example, fumigation is usually carried out before the stalls are re-stocked.
Environmental aspects
Formaldehyde does not accumulate in the environment because it is broken down by sunlight or by bacteria present in the soil or water. Most organisms metabolize formaldehyde quickly and convert it to formic acid so that it does not bioaccumulate .
Emission sources
inside rooms
Certain materials containing formaldehyde, including wood-based materials, floor coverings, furniture and textiles, can contaminate the air we breathe in closed rooms through outgassing . In the 1980s, chipboard and plywood in particular , for the manufacture of which aminoplasts were used as binders, came under suspicion in this context . On the one hand, however, there are many formaldehyde-free bonded wood-based materials and furniture commercially available today. On the other hand, emissions in formaldehyde-based wood-based materials have been significantly reduced. The remediation of buildings contaminated with formaldehyde is still a big issue, especially in older prefabricated wooden houses.
Wood itself emitted formaldehyde through the thermal breakdown of polysaccharides . The emission values depend on the type of wood, the moisture content, the temperature and the storage time of the wood. Fresh oak emits around 430 μg, dry oak around 50 μg formaldehyde per square meter and hour.
Incomplete combustion when smoking produces formaldehyde, which contributes significantly to the pollution of the room air. The total smoke of a single cigarette contains around 0.02–0.1 mg of formaldehyde.
environment
Incomplete combustion processes are an important source of formaldehyde emissions . These can be found, for example, in internal combustion engines in motor vehicles, in foundries and in the manufacture of plastic articles. When bio, sewage and landfill gases are burned in gas engines , high concentrations of formaldehyde are often measured in the exhaust gas. Post-treatment of the exhaust gas is usually necessary so that the emission values do not exceed the statutory limit values.
The combustion of wood in small combustion systems is problematic, as the combustion is often incomplete due to irregular loading or damp wood . In these systems used in house operations, formaldehyde concentrations of 50–100 mg · m −3 arise , which adds up to total emissions of around 1000 tonnes per year for the old federal states (estimate for 1980). The much more productive and cleaner working large industrial combustion plants for the fuels gas , oil and coal had a total emission of only 50 tons per year in 1980. In order to counteract air pollution from chimney stoves and other small combustion systems, the Federal Government stipulated in the ordinance on small and medium-sized combustion systems of January 26, 2010 that only natural wood that has been stored for a sufficiently long time is permitted for combustion.
Emission measurement
Various methods can be used to measure emissions . In the MBTH method , short-chain aliphatic aldehydes, including formaldehyde, are determined in total. For the determination, a partial flow of the laden exhaust gas is brought into reaction with 3-methyl-2-benzothiazolinonhydrazone (MBTH). This creates a blue-colored tetraazapentamethine cyanine cation that can be measured photometrically .
To use the DNPH process , an exhaust gas containing aldehydes and ketones is reacted with 2,4-dinitrophenylhydrazine (DNPH). This can be done either in an absorption solution or on an adsorbent . The resulting 2,4-dinitrophenylhydrazones can then be determined individually using high-performance liquid chromatography and UV detection. If methenamine (urotropine) is contained in the exhaust gas to be sampled, both the DNPH method and the MBTH method lead to increased results due to cross-sensitivities . In this case, the AHMT procedure is recommended . If acrolein and acetaldehyde are suspected in the exhaust gas in addition to formaldehyde , the 2-HMP process can be used, in which the aldehydes contained in the exhaust gas react with 2- (hydroxymethyl) piperidine (2-HMP) and the reaction products are then analyzed by gas chromatography . The acetylacetone process can be used for exhaust gases with a high water content .
The formaldehyde content in the exhaust gas from combustion engines is determined using an automated FTIR method . The exhaust gas to be sampled flows through a measuring cell which is illuminated by infrared radiation. The attenuation of certain wavelengths provides information about the composition of the exhaust gas.
toxicology
In 2012, formaldehyde was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are reassessed and, if necessary, follow-up measures are initiated. Formaldehyde intake was caused by concerns about its classification as a CMR substance , worker exposure , high (aggregated) tonnage and widespread use. The re-evaluation took place from 2013 and was carried out by France . A final report was then published.
acute toxicity
If used improperly, formaldehyde can cause allergies , skin, respiratory tract or eye irritation. There is an acute danger to life (toxic pulmonary edema , pneumonia ) from a concentration of 30 ml / m³. Chronic exposure is carcinogenic and also affects memory, concentration and sleep.
Most poisoning does not occur from direct contact with formaldehyde, but rather from drinking methanol in low-quality alcoholic beverages. The methanol in the body is first converted to formaldehyde by alcohol dehydrogenase , then quickly to formic acid by aldehyde dehydrogenases . This is metabolized slowly and can lead to acidosis . Formaldehyde itself denatures retinal proteins particularly easily , which can lead to blindness. The addition of formaldehyde caused various food scandals. The contaminated foods included pasta, salted fish, tofu, chicken, fruits, and vegetables such as cabbage.
The therapeutic measures for formaldehyde intoxication are diverse. In the case of oral intake, the administration of activated charcoal is advisable (but not milk, which increases the rate of absorption ). The acidosis is treated with an infusion of sodium hydrogen carbonate. Further therapy can be carried out by administering cough sedatives , inhalative β- sympathomimetics or inhalative glucocorticoids . Ammonia vapors neutralize the effects of formalin vapors with the formation of hexamethylenetetraamine .
Carcinogenic risk
Formaldehyde has been legally binding since April 1, 2015 in Annex VI of Regulation 2008/1272 / EC on the classification, labeling and packaging of substances and mixtures in category 1B: “probably carcinogenic in humans”. Formaldehyde has been shown to have carcinogenic effects in animal experiments with rats , but only at high concentrations of 6 ml / m 3 . In 2004 the International Agency for Research on Cancer (IARC) of the World Health Organization changed the classification of formaldehyde, which had existed since 1995, from “suspected carcinogenic effect” to “carcinogenic for humans”. Substances classified as carcinogenic , mutagenic or toxic to reproduction (“CMR substances”) are considered particularly dangerous and must be replaced by less dangerous substances. The background of the WHO classification is an epidemiological study which showed an increased mortality rate from tumors of the nasopharynx in workers who were exposed to formaldehyde in the industry for several years.
The WHO study prompted the Federal Institute for Risk Assessment (BfR) to reassess the cancer-causing risks of formaldehyde. Since 2006, based on the results of its own study , the BfR has considered the carcinogenic effects of formaldehyde to be adequately proven when it is absorbed through the breath. The effect depends on the concentration:
“With indoor air values of or below 124 micrograms of formaldehyde per cubic meter, practically no carcinogenic effects are to be expected. If this value is exceeded repeatedly, there may be health risks. "
A legally binding classification in the Carc 1B category came into force on April 1, 2015.
In the USA , formaldehyde was classified in 1981 in the second report on carcinogens, initially with the suspicion of carcinogenic effects in humans. Since June 2011, the US Department of Health has classified formaldehyde as a carcinogen for humans, as the available studies provide sufficient evidence.
Allergen
For most people, formaldehyde irritation is temporary and reversible, but it can cause allergies. Formaldehyde is a contact allergen . In sensitized people, formaldehyde can cause allergic symptoms even in a concentration of 0.05%. In a patch test series by the North American Contact Dermatitis Group (NACDG) with around 4,500 patients, formaldehyde was found to be the seventh most common allergen, with 9.0% of those tested showing an allergic reaction. The allergic reaction often seen in skin lesions such as skin sores were in the areas that have direct contact with the substance from textiles or cosmetics.
Limit values
According to the CLP regulation , disinfectants containing formaldehyde must be labeled with hazard symbols and warnings such as “May cause an allergic skin reaction”, “Causes severe skin burns and eye damage” or “May cause cancer”. Due to the classification of formaldehyde according to the CLP regulation, the legislators at national and European level have issued various regulations on upper limits for formaldehyde concentration and for labeling products with formaldehyde. The Consumer Goods Ordinance regulates the labeling of detergents and cleaning agents with a concentration of more than 0.1% to 0.2% free formaldehyde.
The Chemicals Prohibition Ordinance prohibits the placing on the market of detergents and cleaning agents with a concentration higher than 0.2% formaldehyde. According to the REACH regulation , a limit value of 300 mg formaldehyde per kilogram applies to clothing, footwear and textiles that do not come into contact with human skin; from November 1, 2023 the limit value will be reduced to 75 mg / kg. The European standard "Safety of Toys" Part 9 (DIN EN 71-9) regulates the content of formaldehyde in toys.
In the field of textiles (clothing), a limit of quantification of 16 mg / kg (16 ppm) applies to voluntary pollutant tests as part of a test seal (e.g. Toxproof or Oeko-Tex 100 ). This is also the limit value for baby clothing. For clothing worn close to the skin, 75 mg / kg apply, for other textiles 300 mg / kg. The permissible “limit value” in Germany is 1500 mg / kg (1500 ppm). This is not a real limit value, as only a note has to be attached that it is recommended to wash the garment for better skin tolerance before wearing it for the first time.
In 2016, the Committee for Indoor Standard Values set a standard value for indoor air of 0.1 mg / m³. In the building industry, a formaldehyde limit value of 120 μg / m³ is defined for buildings with certification according to the German Sustainable Building Council (DGNB) , and if this limit is exceeded, certification is not possible. A target value of 60 μg / m³ is also defined. The working group of ecological research institutes e. V. (AGÖF) has also issued an orientation value for planning of 30 μg / m³.
In March 2015, additions to the legally binding workplace limit values of the TRGS 900 were announced. The value for the maximum workplace concentration of 0.3 ml / m 3 corresponding to 0.37 mg / m 3 , which was recommended by the German Research Foundation (DFG) , was determined.
proof
Detection of free or cleavable formaldehyde is possible with chromotropic acid through the chromotropic acid reaction . Detection is possible with methylbenzothiazolone hydrazone or fuchsin-sulfuric acid ( Schiff's reagent ). Gaseous formaldehyde can also be detected spectroscopically via its absorption in the near UV and in the infrared spectral range. This allows the measurements of formaldehyde concentrations in the earth's atmosphere using remote sensing methods from satellites and from the ground.
The European Pharmacopoeia allows for the limit test for formaldehyde acetylacetone admit. In the acetylacetone process , the formaldehyde reacts with acetylacetone in the presence of ammonium acetate in a Hantzsch dihydropyridine synthesis to form a 3,5-diacetyl-1,4-dihydropyridine derivative, the concentration of which can be determined photometrically .
There are different methods and standards for the quantitative determination of parameters for wood-based materials, which ultimately allow conclusions to be drawn about the emission potential or the "real" emission behavior:
- "Perforator method": Specification in mg formaldehyde per 100 g sample, see Perforator (chemistry)
- Desiccator method: "Small" specimens release formaldehyde into water, given in mg / l
- "Chamber methods": Large panel samples are examined for their formaldehyde emission in a test chamber over a longer period of time , information for the pararosaniline method: ppm with 0.01 ppm = 0.0124 mg formaldehyde per m³ room air = 12.4 µg formaldehyde per m³ Indoor air, limit of quantification 0.01 ppm
The determination takes place according to DIN EN ISO 14184-1: 2011-12 (replacement for DIN 54260: 1988-029), § 64 LFGB (formerly § 35 LMBG) B 82.02-1 (free and releasable formaldehyde) and DIN EN 717- 1 (wood-based materials, formaldehyde release according to the test chamber method) or according to DIN EN 120 (wood-based materials - determination of the formaldehyde content according to the perforator method).
literature
- Luoping Zhang: Formaldehyde. Exposure, Toxicity and Health Effects. Royal Society of Chemistry, London 2018, ISBN 978-1-78262-973-3 .
- Wilhelm Keim : Plastics. Synthesis, manufacturing processes, equipment. Wiley-VCH, Weinheim 2006, ISBN 3-527-31582-9 .
Web links
![]() Wikisource: Formalin - Wikisource contains the text ofthe 1911 Encyclopædia Britannicaarticle Formalin .
Wikisource: Formalin - Wikisource contains the text ofthe 1911 Encyclopædia Britannicaarticle Formalin .
Individual evidence
- ^ Roche Lexicon Medicine. 4th edition, Urban & Schwarzenberg 1998, ISBN 3-541-17114-6 .
- ↑ a b c d e f g h Entry on formaldehyde in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ^ CRC Handbook of Chemistry and Physics. 85th Edition, CRC Press, Boca Raton 2004.
- ↑ a b c Entry on formaldehyde in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 50-00-0 or formaldehyde ), accessed on September 13, 2019.
- ↑ a b Entry on formaldehyde in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on October 17, 2016.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-19.
- ^ A b Lars Axelsen: Outlook for Formaldehyde and Impact on Methanol Demand. Presentation at the 33rd Annual IHS Chemical World Methanol Conference; 11-12. November 2015, Munich.
- ^ A b C. H. Fox, FB Johnson, J. Whiting and PP Roller: Formaldehyde Fixation. In: Journal of Histochemistry and Cytochemistry . 1985, Vol. 33, No. 8, pp. 845-853.
- ↑ Reinhard Hildebrand: Formalin. In: Werner E. Gerabek , Bernhard D. Haage, Gundolf Keil , Wolfgang Wegner (eds.): Enzyklopädie Medizingeschichte. de Gruyter, Berlin / New York 2005, ISBN 3-11-015714-4 , p. 410.
- ↑ Patent US3816539A : Method for making a stabilized solution of concentrated aqueous formaldehyde. Published December 18, 1968 , Inventors: J. Sanborn, W. Lemmons, J. Ramey.
- ↑ Patent US656061A : Process of producing formaldehyde vapors. Published November 24, 1896 , inventor: Jean Joseph Auguste Trillat.
- ↑ a b c Günther Brugge: From the early history of formaldehyde production. In: Chemical Apparatus . 1931, pp. 157-160.
- ↑ Adolf Baeyer: About the compounds of aldehydes with phenols and aromatic hydrocarbons. In: Reports of the German Chemical Society . 1872, Vol. 5, No. 2, pp. 1094-1100.
- ^ A b c d e Günther Reuss, Walter Disteldorf, Armin Otto Gamer, Albrecht Hilt: Formaldehyde. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2012, ISBN 978-3-527-32943-4 . Pp. 735-768.
- ^ A b Günther Brugge: From the early history of formaldehyde and its applications. In: Chemical technology . 1943, Volume 16, pp. 228-230.
- ↑ G. Greco, U. Soldano: New plants for the production of formaldehyde from methanol. In: Chemical Engineer Technology . 1959, Volume 31.12, pp. 761-765.
- ↑ Oliver Türk: Material use of renewable raw materials. Springer-Verlag / Vieweg, Wiesbaden 2014, ISBN 978-3-8348-1763-1 , pp. 130-133.
- ^ Fred Aftalion: A History of the International Chemical Industry. Chemical Heritage Foundation, Philadelphia, ISBN 0-941901-29-7 , p. 118.
- ^ Fred Aftalion: A History of the International Chemical Industry. Chemical Heritage Foundation, Philadelphia, ISBN 0-941901-29-7 , p. 149.
- ^ Friedrich Asinger : methanol, chemical and energy raw material. Akademie-Verlag, Berlin 1987, ISBN 3-05-500341-1 , p. 5.
- ↑ M. Klar: Chemical products made from wood by dry distillation. In: A look around in science and technology . 1938, pp. 741-742.
- ^ Boy Cornils, Wolfgang A. Herrmann, M. Muhler, C. Wong: Catalysis from A to Z: A Concise Encyclopedia. Wiley-VCH, 2007, ISBN 978-3-527-31438-6 , p. 27.
- ↑ Homer Adkins, Wesley R. Peterson: The Oxidation of Methanol with Air over Iron, Molybdenum, and Iron-Molybdenum Oxides. In: Journal of the American Chemical Society. Volume 53, 1931, pp. 1512-1520, doi: 10.1021 / ja01355a050 .
- ↑ Jorge Prieto, Jürgen Keine: Wood coating. Vincentz Network, 2019, ISBN 978-3-748-60171-5 , p. 41.
- ↑ a b c European Food Safety Authority: Endogenous formaldehyde turnover in humans compared with exogenous contribution from food sources. In: EFSA Journal . Volume 12, 2014, pp. 3550-3561, doi: 10.2903 / j.efsa.2014.3550 .
- ^ Toxic Exposures. Second edition. Lippincott Williams and Wilkins, Philadelphia, Pennsylvania 1999, p. 1008.
- ^ A b c d Ursula Wiedermann-Schmidt and Wolfgang Maurer: Auxiliaries and additives of vaccines - Medical relevance . In: Wiener Klinische Wochenschrift . tape 117 , no. 15 , August 1, 2005, ISSN 1613-7671 , p. 510-519 , doi : 10.1007 / s00508-005-0405-0 .
- ^ Peder Wolkoff, Gunnar D. Nielsen: Non-cancer effects of formaldehyde and relevance for setting an indoor air guideline. In: Environment International . Volume 36, 2010, pp. 788-799.
- ↑ I. Kushch et al. a .: Compounds enhanced in a mass spectrometric profile of smokers' exhaled breath versus non-smokers as determined in a pilot study using PTR-MS. In: Journal of breath research . 2008, PMID 21383443 .
- ↑ T. Szarvas et al. a .: Determination of endogenous formaldehyde level in human blood and urine by dimedone- 14 C radiometric method. In: Journal of Radioanalytical and Nuclear Chemistry . Volume 106, No. 6, 1986, pp. 357-367.
- ^ Richard S. Hanson, Thomas E. Hanson: Methanotrophic Bacteria. In: Microbiological Reviews . 1996, Vol. 60, No. 2, pp. 439-471.
- ↑ R. Paul Jensen, Wentai Luo, James F. Pankow, Robert M. Strongin, David H. Peyton: Hidden Formaldehyde in E-Cigarette aerosol. In: New England Journal of Medicine . Volume 372, 2015, pp. 392-394, doi: 10.1056 / NEJMc1413069 .
- ↑ BJ Vorath, D. Steffens: Safety and health protection when smoking. Ergonomic findings No. 113. Federal Institute for Occupational Safety and Health, Dortmund, 1999, ISSN 0720-1699 .
- ↑ Chuan Wang, Xiao-Feng Huang, Y. and Han, B. o. Zhu, Ling-Yan He: Sources and Potential Photochemical Roles of Formaldehyde in an Urban Atmosphere in South China. In: Journal of Geophysical Research . Atmospheres. Volume 122, 2017, pp. 11934-11947, doi: 10.1002 / 2017JD027266 .
- ↑ DJ Luecken, SL Napelenok, M. Strum, R. Scheffe, S. Phillips: Sensitivity of Ambient Atmospheric Formaldehyde and Ozone to Precursor Species and Source Types Across the United States. In: Environmental Science & Technology . Volume 52, 2018, pp. 4668-4675, doi: 10.1021 / acs.est.7b05509 .
- ^ Samuel G. Thomas, William A. Guillory: Condensed-phase photochemistry of formaldehyde. In: Journal of Physical Chemistry . Volume 77, 1973, pp. 2469-2472, doi: 10.1021 / j100639a021 .
- ↑ E. Peters, F. Wittrock, K. Großmann, U. Frieß, A. Richter, JP Burrows: Formaldehyde and nitrogen dioxide over the remote Western Pacific Ocean: SCIAMACHY and GOME-2 validation. In: Atmospheric Chemistry and Physics Discussions . Volume 12, 2012, p. 15977, doi: 10.5194 / acpd-12-15977-2012 .
- ↑ Donald J. Wuebbles, Katharine Hayhoe: Atmospheric methane and global change. In: Earth Science Reviews . Volume 57, No. 3, 2002, pp. 177-210.
- ↑ Joseph P. Pinto, G. Randall Gladstone, Yuk Ling Yung: Photochemical Production of Formaldehyde in Earth's Primitive Atmosphere. In: Science . 1980, pp. 183-185, doi: 10.1126 / science.210.4466.183 .
- ↑ Benjamin Zuckerman , D. Buhl, P. Palmer, LE Snyder: Observations of Interstellar Formaldehyde. In: Astrophysical Journal . 1970, vol. 160, pp. 485-506.
- ↑ JG Mangum, J. Darling, KM Menten, C. Henkel: Formaldehyde densitometry of starburst galaxies. In: Astrophysical Journal . 2008, Volume 673, pp. 832-846.
- ↑ C. Henkel, R. Guensten, FF Gardner: [ 12 C] / [ 13 C] ratios from formaldehyde in the inner galactic disc. In: Astronomy and Astrophysics . 1985, Vol. 143, pp. 148-152.
- ↑ Markus Schuhmann u. a .: CHO-Bearing Molecules in Comet 67P / Churyumov-Gerasimenko. In: ACS Earth and Space Chemistry . Volume 3, 2019, pp. 1854–1861, doi: 10.1021 / acsearthspacechem.9b00094 .
- ↑ E. Zubritsky, N. Neal-Jones: RELEASE 14-038 - NASA's 3-D Study of Comets Reveals Chemical Factory at Work .
- ↑ MA Cordiner u. a .: Mapping the release of volatiles in the inner coma of comets C / 2012 F6 (Lemmon) AND C / 2012 S1 (Ison) using the Atacama large millimeter / submillimeter array. In: The Astrophysical Journal . Volume 792, 2014, p. L2, doi : 10.1088 / 2041-8205 / 792/1 / L2 .
- ↑ GD Cody, E. Heying, CM Alexander, LR Nittler, AL Kilcoyne, SA Sandford, RM Stroud: Establishing a relationship between molecular chondritic and cometary organic solids. In: Proceedings of the National Academy of Sciences . Volume 108, number 48, November 2011, pp. 19171-19176, doi: 10.1073 / pnas.1015913108 , PMID 21464292 , PMC 3228457 (free full text).
- ^ Luoping Zhang: Introduction to Formaldehyde. In: Formaldehyde: Exposure, Toxicity and Health Effects. Royal Society of Chemistry, 2018, ISBN 978-1-78262-973-3 , pp. 1-19.
- ↑ a b c d e f g h Hans-Jürgen Arpe : Industrial organic chemistry. Important preliminary and intermediate products . 6th edition. Wiley-VCH, Weinheim 2007, ISBN 978-3-527-31540-6 , pp. 41-43 .
- ↑ a b Formaldehydes exceed 52 million tons.
- ↑ Manfred Baerns , Arno Behr , Axel Brehm, Jürgen Gmehling , Kai-Olaf Hinrichsen, Hanns Hofmann , Regina Palkovits, Ulfert Onken, Albert Renken: Technische Chemie . 2nd Edition. Wiley-VC, Weinheim 2013, ISBN 978-3-527-33072-0 , pp. 594 .
- ^ Graeme J. Millar, Mary Collins: Industrial Production of Formaldehyde Using Polycrystalline Silver Catalyst. In: Industrial & Engineering Chemistry Research. Volume 56, 2017, pp. 9247-9265, doi: 10.1021 / acs.iecr.7b02388 .
- ^ Roland Dittmeyer, Wilhelm Keim, Gerhard Kreysa, Alfred Oberholz (eds.): Winnacker • Küchler: Chemical technology - processes and products - organic intermediate compounds, polymers . 5th edition. tape 5 . Wiley-VCH, Weinheim 2005, ISBN 978-3-527-30770-8 .
- ^ Adam W. Franz, Helmut Kronemayer, Daniel Pfeiffer, Roman D. Pilz, Gänther Reuss, Walter Disteldorf, Armin Otto Gamer, Albrecht Hilt: Formaldehyde. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley ‐ VCH, November 22, 2016, doi : 10.1002 / 14356007.a11_619.pub2 .
- ^ Friedrich Asinger : Chemistry and technology of paraffin hydrocarbons. Akademie-Verlag, Berlin 1956, pp. 477-484.
- ↑ Patent US2977386 : Absorption of formaldehyde in alkaline urea solution .. Published on March 28, 1961 , inventor: Mearl A. Kise.
- ↑ E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ VDI heat atlas. 9th edition, Springer-Verlag, Berlin 2002, ISBN 978-3-662-10744-7 , DA 7.
- ↑ S.-X. Weng, BH Torrie, BM Powell: The crystal structure of formaldehyde. In: Molecular Physics . Volume 68, 1989, pp. 25-31, doi: 10.1080 / 00268978900101941 .
- ^ DJ Clouthier, DA Ramsay: The Spectroscopy of Formaldehyde and Thioformaldehyde. In: Ann. Rev. Phys. Chem. 1983, Vol. 34, pp. 31-58.
- ↑ a b K. S. Tewari, NK Vishnoi: A Textbook of Organic Chemistry. Vikas, 2017, ISBN 978-81259-1605-5 , p. 165.
- ↑ B. Tollens: About ammoniacal silver solution as a reagent for aldehyde. In: Reports of the German Chemical Society . 1882, Vol. 15, No. 2, pp. 1635-1639.
- ^ L. Henry: Sur le nitrile glycolique et la synthèse directe de l'acide glycolique. In: Comp. Rend. 1890, vol. 110, pp. 759-760.
- ↑ Alexander Butlerow : About some derivatives of iodomethylene. In: Annals of Chemistry and Pharmacy . Volume 111, 1859, pp. 242-252, doi: 10.1002 / jlac.18591110219 .
- ↑ C. Mannich, W. Krösche: About a condensation product of formaldehyde, ammonia and antipyrine. In: Archives of Pharmacy . 1912, Volume 250, pp. 647-667, doi: 10.1002 / ardp.19122500151 .
- ↑ E. Müller. O. Bayer. H. Meerwein, K. Ziegler: Houben-Weyl Methods of Organic Chemistry Vo. X / 1, 4th edition. Nitro, Nitroso and Hydroxylamine Compounds. Thieme, Stuttgart, 1971, ISBN 978-3-13-209104-7 , p. 254.
- ↑ Whitmore FC et al. a .: Production of Benzyl Chloride by Chloromethylation of Benzene. Laboratory and Pilot Plant Studies. In: Industrial & Engineering Chemistry . Volume 38, 1946, pp. 478-485, doi: 10.1021 / ie50437a013 .
- ↑ a b Friedrich Asinger : Methanol, chemical and energy raw material. Akademie-Verlag, Berlin 1987, ISBN 3-05-500341-1 , pp. 437-438.
- ^ Friedrich Asinger : methanol, chemical and energy raw material. Akademie-Verlag, Berlin 1987, ISBN 3-05-500341-1 , p. 348.
- ^ Ronald Breslow : On the mechanism of the formose reaction. In: Tetrahedron Letters . Volume 1, 1959, pp. 22-26, doi: 10.1016 / S0040-4039 (01) 99487-0 .
- ↑ Entry on formaldehyde. In: Römpp Online . Georg Thieme Verlag, accessed on February 28, 2014.
- ^ Manfred Dunky, Peter Niemz: Wood-based materials and glues: Technology and influencing factors. Springer-Verlag, Berlin / Heidelberg / New York 2002, ISBN 3-540-42980-8 , pp. 249-302.
- ^ Manfred Dunky, Peter Niemz: Wood-based materials and glues: Technology and influencing factors. Springer-Verlag, Berlin / Heidelberg / New York 2002, ISBN 3-540-42980-8 , pp. 303-320.
- ↑ Hermann Rath: Textbook of textile chemistry, including textile-chemical technology. Springer-Verlag, Berlin / Heidelberg 1963, ISBN 978-3-662-00065-6 , pp. 112-121.
- ↑ Hans-Jürgen Bargel, Hermann Hilbrans, Günter Schulze, Karl-Heinz Hübner, Oswald Krüger: Material science. Springer-Verlag, Berlin / Heidelberg 2005, ISBN 978-3-540-26107-0 , p. 386.
- ^ WC Bauman, J. Eichhorn: Fundamental Properties of a Synthetic Cation Exchange Resin. In: Journal of the American Chemical Society. Volume 69, 1947, pp. 2830-2836, doi: 10.1021 / ja01203a065 .
- ^ Wilhelm Keim : plastics. Synthesis, manufacturing processes, equipment. Chapter 4, Wiley-VCH, Weinheim 2006, ISBN 3-527-31582-9 .
- ↑ a b Manfred Baerns , Arno Behr , Axel Brehm, Jürgen Gmehling , Kai-Olaf Hinrichsen, Hanns Hofmann , Regina Palkovits, Ulfert Onken, Albert Renken: Technische Chemie. Wiley-VCH, Weinheim 2013, ISBN 978-3-527-33072-0 , p. 590.
- ^ Isocyanates production: Global and Russian Isocyanate Market Overview.
- ↑ Regulation (EU) 2019/831 .
- ↑ Regulation (EC) No. 1223/2009 .
- ^ Stanley A. Plotkin et al: Plotkin's Vaccines . 7th edition. Elsevier, Philadelphia 2017, ISBN 978-0-323-35761-6 , pp. 1594 .
- ^ Vaccines for human use. In: European Pharmacopeia . Volume 8.0, p. 767.
- ^ Vaccines for veterinary use. In: European Pharmacopeia . Volume 8.0, p. 770.
- ↑ a b K. Weißer, I. Barth, B. Keller-Stanislawski: Safety of vaccines . In: Federal Health Gazette - Health Research - Health Protection . tape 52 , no. 11 , November 1, 2009, ISSN 1437-1588 , p. 1053-1064 , doi : 10.1007 / s00103-009-0961-y .
- ↑ Wolfgang Maurer: Vaccine skeptics - vaccine opponents. From another reality on the internet . In: Pharmacy in our time . tape 37 , no. 1 , 2008, p. 64-70 , doi : 10.1002 / pauz.200700252 .
- ^ Heinz Fraenkel-Conrat , BA Brandon, HS Olcott: The reaction of formaldehyde with proteins; participation of indole groups; gramicidin. In: Journal of biological chemistry . Volume 168, Number 1, April 1947, pp. 99-118, PMID 20291066 .
- ↑ Heinz Fraenkel-Conrat, HS Olcott: The reaction of formaldehyde with proteins. Cross-linking between amino and primary amide or guanidyl groups. In: Journal of the American Chemical Society . Volume 70, Number 8, August 1948, pp. 2673-2684, PMID 18876976 .
- ^ Heinz Fraenkel-Conrat, HS Olcott: Reaction of formaldehyde with proteins. Cross-linking of amino groups with phenol, imidazole, or indole groups. In: Journal of biological chemistry . Volume 174, Number 3, July 1948, pp. 827-843, PMID 18871242 .
- ↑ Rose-Maria Gropp: Unfrischer Fisch. In: FAZ.net. June 29, 2006.
- ^ State Institute for Work Design of the State of North Rhine-Westphalia . Retrieved May 19, 2018.
- ↑ Medical device manufacturer MEDIS . Retrieved May 19, 2018.
- ^ R. Coleman, I. Kogan: An improved low-formaldehyde embalming fluid to preserve cadavers for anatomy teaching. In: J. Anat. 192, 1998, pp. 443-446.
- ↑ J. Weigner: Nitrite curing salt-ethanol-polyethylene glycol 400 solution for the fixation and preservation of organs and animal bodies for teaching and research. In: The taxidermist . Vol. 57, 2011, pp. 34-53, ISSN 0032-6542 .
- ^ W. Thiel: The preservation of whole corpses in natural colors. In: Ann. Anat. 174, 1992, pp. 185-195.
- ^ Rüdiger Kramme: Medical technology: procedures - systems - information processing. Springer Medizin Verlag, Heidelberg 2007, ISBN 978-3-540-34102-4 , pp. 27-28.
- ↑ Michael Berger, Lutz Nitschke: Measurement of formaldehyde in chicken coop fumigation. In: Hazardous substances - cleanliness. Air . Volume 75, No. 4, 2015, pp. 127-132.
- ↑ MZM Salem, M. Böhm: Understanding of formaldehyde emissions from solid wood: An overview. In: BioRes . 2013, pp. 4775-4790.
- ↑ Formaldehyde problems with gas engines. SGS-RUK GmbH, accessed on February 5, 2014.
- ↑ BGBl. I pp. 804, 828
- ↑ Federal Ministry for the Environment, Nature Conservation and Nuclear Safety: Heating with wood. The right fuel.
- ↑ VDI 3862 sheet 1: 1990-12 measurement of gaseous emissions; Measurement of aliphatic aldehydes (C 1 to C 3 ) using the MBTH method (Gaseous emission measurement; measurement of aliphatic aldehydes (C 1 to C 3 ) MBTH method). Beuth Verlag, Berlin, pp. 2–3.
- ↑ a b VDI 3862 sheet 2: 2000-12 measurement of gaseous emissions; Measuring aliphatic and aromatic aldehydes and ketones by the DNPH method; Gas washing bottle method (Gaseous emission measurement; Measurement of aliphatic and aromatic aldeydes and ketones by DNPH method; Impinger method). Beuth Verlag , Berlin, pp. 4–5.
- ↑ VDI 3862 sheet 3: 2000-12 measurement of gaseous emissions; Measuring aliphatic and aromatic aldehydes and ketones by the DNPH method; Cartridge method (Gaseous emission measurement; Measurement of aliphatic and aromatic aldeydes and ketones by DNPH method; Cartridges method). Beuth Verlag, Berlin, p. 5.
- ^ Franz Joseph Dreyhaupt (ed.): VDI-Lexikon Umwelttechnik. VDI-Verlag Düsseldorf 1994, ISBN 3-18-400891-6 , p. 515.
- ^ A b Hans-Günter Haub, Sigrid Mühlhauser, Franz-Josef Müller, Arno Gardziella: Measurement of emissions during the curing of phenolic resins. In: Dust - cleanliness. Air . Volume 48, No. 4, 1988, pp. 145-149.
- ↑ VDI 3862 sheet 4: 2001-05 measurement of gaseous emissions; Measurement Measurement of formaldehyde by the AHMT method (Gaseous emission measurement; Measurement of formaldehyde by the AHMT method). Beuth Verlag, Berlin, p. 5.
- ↑ VDI 3862 sheet 5: 2008-06 measurement of gaseous emissions; Measurement of lower aldehydes especially acrolein with the 2-HMP method - GC method (Gaseous emission measurement; Measurement of lower aldehydes especially acrolein with the 2-HMP method - GC method). Beuth Verlag, Berlin, p. 4.
- ↑ VDI 3862 sheet 6: 2004-02 measurement of gaseous emissions; Measurement of formaldehyde by the acetylacetone method (Gaseous emission measurement; Measurement of formaldehyde by the acetylacetone method). Beuth Verlag, Berlin, p. 5.
- ↑ VDI 3862 sheet 8: 2015-06 measurement of gaseous emissions; Measurement of formaldehyde in exhaust gas from internal combustion engines; FTIR method (Measurement of gaseous emissions; Measurement of formaldehyde in the exhaust gas of combustion engines; FTIR method). Beuth Verlag, Berlin, pp. 3-4.
- ↑ European Chemicals Agency (ECHA): Substance Evaluation Conclusion and Evaluation Report.
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Formaldehyde , accessed on May 1, 2020.
- ^ Info page formaldehyde of the Institute for Applied Environmental Research eV
- ^ Lutz Edler, DKFZ - German Cancer Research Center, Heidelberg: Formaldehyde - The history of a chemical in animal experiments, studies in humans and risk assessments. (PDF; 353 kB) bfr.bund.de, accessed on June 6, 2009 .
- ↑ Made from China . In: Der Spiegel . No. 42 , 2012 ( online ).
- ↑ World Health Organization WHO : IARC classifies formaldehyde as carcinogenic to humans. ( Memento from September 10, 2018 in the Internet Archive ). Press release 153, June 15, 2004.
- ↑ BfR : Formaldehyde - more dangerous than previously thought? (PDF; 59 kB), statement of November 29, 2004.
- ↑ BfR : Toxicological assessment of formaldehyde. (PDF; 111 kB), Opinion 023/2006, March 30, 2006.
- ↑ BfR : Carcinogenic effects of inhaled formaldehyde have been sufficiently proven. Press release 14/2006, May 29, 2006.
- ^ National Institute of Environmental Health Sciences. 13th Report on Carcinogens (RoC): Formaldehyde. Retrieved November 18, 2014.
- ↑ Kathryn A. Zug u. a .: Patch-Test Results of the North American Contact Dermatitis Group 2005-2006. In: dermatitis. Volume 20, 2009, pp. 149-160, doi: 10.2310 / 6620.2009.08097 .
- ↑ Commodities Ordinance .
- ↑ ChemVerbotsV, Annex 1 Bans on placing on the market.
- ↑ Regulation (EU) 2018/1513 of the Commission dated October 10, 2018 amending Annex XVII of Regulation (EC) No. 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) with regard to certain substances classified as carcinogenic, germ cell mutagenic or toxic to reproduction (CMR) of category 1A or 1B .
- ↑ Announcement of the Federal Environment Agency. Guide value for formaldehyde in indoor air. August 2016 (PDF; 127 kB).
- ↑ Formaldehyde in the room air. Background information. In: ee-concept.de. Retrieved April 11, 2014
- ↑ AGÖF orientation values for volatile organic compounds in indoor air 2013. Arbeitsgemeinschaft Ökologischer Forschungsinstitute e. V. (AGÖF), accessed on April 11, 2014.
- ↑ Changes and new additions to the TRGS 900 "Occupational Exposure Limits" .
- ↑ Johannes Orphal , Kelly Chance: Ultraviolet and visible absorption cross-sections for HITRAN. In: Journal of Quantitative Spectroscopy and Radiative Transfer . Volume 82, No. 1, 2003, pp. 491-504.
- ^ I. De Smedt, u. a .: Twelve years of global observations of formaldehyde in the troposphere using GOME and SCIAMACHY sensors. In: Atmospheric Chemistry and Physics . Volume 8, 2008, pp. 4947-4963.
- ↑ E. Peters et al. a .: Formaldehyde and nitrogen dioxide over the remote western Pacific Ocean: SCIAMACHY and GOME-2 validation using ship-based MAX-DOAS observations. In: Atmospheric Chemistry and Physics. Volume 12, No. 22, 2012, pp. 11179-11197.
- ↑ VDI 3484 sheet 2: 2001-11: Measurement of gaseous immissions; Measuring indoor air pollution; Determination of the formaldehyde concentration by the acetylacetone method (Gaseous ambient air measurements; Indoor-air pollution measurements; Measurement of the formaldehyde concentration with the acatylacetone method). Beuth Verlag, Berlin, p. 4.
















![{\ displaystyle \ mathrm {CH_ {2} O + 2 \ [Ag (NH_ {3}) _ {2}] ^ {+} + 2 \ OH ^ {-} \ longrightarrow}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5f144ec092e566bf5d594c572f95177feb09d9e7)