Hexamethylenetetramine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
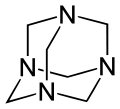
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Methenamine | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 6 H 12 N 4 | ||||||||||||||||||
| Brief description |
colorless crystals with an amine-like odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 140.19 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.33 g cm −3 (20 ° C) |
||||||||||||||||||
| boiling point |
263 ° C sublimates with partial decomposition |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Hexamethylenetetramine (also methenamine or urotropine ) is a colorless crystalline powder. The chemical structure of hexamethylenetetramine can be derived from the hydrocarbon adamantane : In hexamethylenetetramine, nitrogen atoms are located at the four connecting points of the three six-membered rings . Between the nitrogen atoms (as the corner points of a tetrahedron ) there is a CH 2 group .
Hexamethylenetetramine was first described by Alexander Michailowitsch Butlerow in 1859 . In 1894 it was introduced into therapy under the brand name Urotropin for disinfecting the urinary tract.
Manufacturing
Hexamethylenetetramine is formed from ammonia and formaldehyde in an aqueous solution:
This reaction is used in the so-called formol titration for the determination of ammonium compounds .
properties
The flash point is 250 ° C, the ignition temperature 390 ° C and the decomposition temperature> 263 ° C. The energy density , calorific value is 31.3 MJ / kg, the pH value is 8.4 at 28 g / l and the pKs value is 8.95 (20 ° C)
use
Hexamethylenetetramine is used in the production of amino and phenoplasts and as a food preservative (E 239, rarely used, only permitted in provolone cheese). In pressed form, it is also used as a dry fuel and is the main component of Esbit fuel - in the form of cuboid tablets with gray marks.
In histochemistry, hexamethylenetetramine is used for silver staining .
In organic synthesis it serves as a formyl equivalent ( Duff reaction ), for the introduction of amino groups , for the synthesis of N - heterocycles and is used in the Mannich reaction . In inorganic analysis, it serves cations - separation process as buffer substance in the precipitation of the " Urotropin group " (which includes iron , chromium and aluminum are) at pH 5.5. Methenamine breaks down into formaldehyde and ammonium ions in an acidic aqueous solution . For this reason, sublimed hexamethylenetetramine leads to excessive formaldehyde concentrations when measuring emissions during the production of phenolic resins in measuring processes that work with acidic solutions. In this context, the DNPH method and the MBTH method should be mentioned.
Hexamethylenetetramine is a starting material for the production of the explosives hexogen , octogen , dinitrohexamine and HMTD . As such, it is subject to Regulation (EU) No. 98/2013 on the marketing and use of precursors for explosives (Annex II).
In foundry technology, it is used together with phenolic resin as a resin-hardener system for the mask molding process for the production of shell molds and hollow cores.
Another use is the neutralization of acidic by-products in the synthesis of the chemical warfare agent sarin , which is why the detection of hexamine (= hexamethylenetetramine) in combat areas can be considered an indication of the use of sarin.
Hexamethylenetetramine (non-proprietary name: methenamine ) is used medically against excessive perspiration , e.g. B. on the hands, feet or armpits (trade name: Antihydral ). In its use as a disinfectant for urinary tract infections, methenamine ( urotropin ) has been replaced by antibiotics . The effect is based on the elimination of formaldehyde in an acidic environment, which is why it was combined with uric acidic substances ( camphoric acid , anhydromethylene citric acid, etc.).
Individual evidence
- ↑ entry to methenamine in CosIng database of the European Commission, accessed on 28 December of 2019.
- ↑ a b c d e f g Entry on hexamethylenetetramine in the GESTIS substance database of the IFA , accessed on December 16, 2019(JavaScript required) .
- ↑ a b c d e f Entry on hexamethylenetetramine. In: Römpp Online . Georg Thieme Verlag, accessed on July 10, 2019.
- ↑ a b European Pharmacopoeia, 8th edition, Grundwerk 2014, p. 4065.
- ↑ Entry on Methenamine in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Alexander Butlerow : About some derivatives of iodomethylene. In: Annals of Chemistry and Pharmacy . 111, 1859, pp. 242-252, doi: 10.1002 / jlac.18591110219 .
- ^ A b H. Auterhoff: Textbook of Pharmaceutical Chemistry . WVG 1978, p. 179.
- ↑ VDI 3862 sheet 2: 2000-12 measurement of gaseous emissions; Measuring aliphatic and aromatic aldehydes and ketones by the DNPH method; Gas washing bottle method (Gaseous emission measurement; Measurement of aliphatic and aromatic aldeydes and ketones by DNPH method; Impinger method). Beuth Verlag, Berlin, p. 4.
- ↑ Hans-Günter Haub, Sigrid Mühlhauser, Franz-Josef Müller, Arno Gardziella: Measurement of emissions during the curing of phenolic resins. In: Dust - cleanliness. Air . 48, No. 4, 1988, pp. 145-149.
- ↑ According to the news magazine Spiegel, a report by the French foreign ministry said: "In addition, the presence of hexamine indicates that this manufacturing process was developed by the science and research center of the Syrian regime." Syrian war: France blames Assad for sarin attack . In: Spiegel Online . April 26, 2017 ( spiegel.de [accessed November 1, 2017]).
- ↑ Christoph Sydow: Chemical weapons in Syria: Assad regime itself provides evidence of sarin attack . In: Spiegel Online . July 5, 2017 ( spiegel.de [accessed November 1, 2017]).
- ↑ Red List online, as of December 12, 2009.
- ↑ Ch. Weber: Hyperhidrosis - when the sweat breaks out . DAZ.online , No. 31/2006 .
- ^ H. Auterhoff: Dictionary of Pharmacy . Volume 1: Pharmaceutical Biology, Pharmaceutical Chemistry, Pharmaceutical Technology . Wissenschaftliche Verlagsgesellschaft, Stuttgart 1981, ISBN 3-8047-0656-8 , p. 262.



