Silver coloring
The silver staining is a procedure for staining of proteins , carbohydrates , DNA and RNA in polyacrylamide gels after electrophoresis or in thin sections of tissues .
history
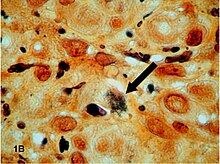
The process is based on the development of photographs. This developed in the 19th century from Thomas Wedgwood's photograms , followed by the daguerreotype and the calotype . From 1873 onwards, Camillo Golgi used the Golgi stain under the name “la reazione nera” (in German “the black reaction”) to contrast the nervous system in tissue sections, later it was also known as the Golgi method .
Applications

Molecules in biological tissues that bind more silver ions are known as argyrophilic . There are also argent-affine molecules that bind and also reduce silver ions .
histology
Today, the methenamine- based silver stains according to Grocott (for various mushrooms ), according to Gömöri , according to Warthin-Starry (stains Spirochaeten , Helicobacter pylori , Lawsonia intracellularis , Microsporidia ) according to Dieterle (stains Bartonella henselae , Treponema pallidum ) are used for silver staining of tissues and Mycobacterium tuberculosis ) or according to Jones (stains basement membranes in the diagnosis of glomerulonephritis ).
In Von Kossa staining , the silver ions are precipitated by phosphates . Subsequent reduction with hydroquinone creates black-brown, elemental silver. Thus, the formation of hydroxyapatite z. B. be stained in osteoblasts .
biochemistry
The silver staining of proteins in agarose gels was developed in 1973 by Kerenyi and Gallyas and has since been optimized for polyacrylamide gels, and also for the staining of DNA or RNA. The glycosylations of glycoproteins and polysaccharides can be oxidized by pretreating them with a 0.1% solution of periodic acid at 4 ° C for one hour , so that the storage of silver ions can be improved.
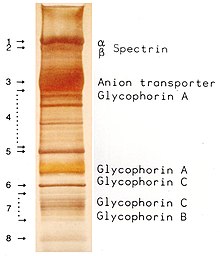
First, the proteins are denatured in the gel by a fixing solution of 10% glacial acetic acid and 30% ethanol and precipitate, while the detergent (mostly SDS ) is removed at the same time . The diffusion of the proteins is thus significantly reduced. After washing the gel several times with water, it is incubated in a silver nitrate solution. Silver ions attach themselves to negatively charged side chains of the proteins. Excess silver is then washed off with water. In the final development step, the silver ions are reduced to elemental silver by adding alkaline formaldehyde . This colors the places where proteins are present black.
The intensity of the color depends on the primary structure of the protein. The cleanliness of the vessels used and the purity of the reagents also affect the silver color. Frequent artifacts are bands of keratin in the range from 54 to 57 kDa and from 65 to 68 kDa, which was also separated as contamination of the sample in the electrophoresis.
This method is characterized by its high sensitivity compared to Coomassie-stained gels or other protein stains . The detection limit is 0.1 ng to 1 ng per band.
Argyria
The argyria is a condition defined by regular intake of silver salt or silver colloid can arise and in a gray discoloration of the skin, night blindness and kidney failure can express.
literature
- Gold and Silver Staining: Techniques in Molecular Morphology . In: Gerhard W. Hacker, Jiang Gu (Eds.): Advances in Pathology, Microscopy, & Molecular Morphology . CRC Press, Boca Raton 2002, ISBN 1-4200-4023-5 (English, 264 pages, limited preview in Google Book Search).
Individual evidence
- ↑ E. Hempelmann, O. Götze: Characterization of membrane proteins by polychromatic silver staining . In: Hoppe Seyler's Z Physiol Chem . tape 365 , 1984, pp. 241-242 .
- ↑ T. Wedgwood, H. Davy: An account of a method of copying paintings upon glass and making profiles by the agency of light upon nitrate of silver, invented by T. Wedgwood, Esq., With observations by H. Davy. In: Journal of the Royal Institution. Volume 1, No. 9, London, June 22, 1802.
- ^ G. Grant: How the 1906 Nobel Prize in Physiology or Medicine was shared between Golgi and Cajal . In: Brain Res Rev . tape 55 , no. 2 , October 2007, p. 490-498 , doi : 10.1016 / j.brainresrev.2006.11.004 , PMID 17306375 .
- ^ C. Golgi: Sulla struttura della sostanza grigia del cervello. In: Gazzetta Medica Italiana (Lombardia) . tape 33 , 1873, pp. 244-246 .
- ^ RG Grocott: A stain for fungi in tissue sections and smears using Gomori's methenamine-silver nitrate technic. In: American journal of clinical pathology. Volume 25, Number 8, August 1955, pp. 975-979, ISSN 0002-9173 . PMID 14398663 .
- ↑ G. Gömöri: A new histochemical test for glycogen and mucin. In: American Journal of Clinical Pathology. (1946), Volume 16, p. 177.
- ↑ AS Warthin, AC Chronister: A more rapid and improved method of demonstrating spirochetes in tissues (Warthin and Starry's cover-glass method). In: American Journal of Syphilis. 4, 1920, pp. 97-103.
- ↑ B. Huerta, A. Arenas, L. Carrasco, A. Maldonado, C. Tarradas, A. Carbonero, A. Perea: Comparison of diagnostic techniques for porcine proliferative enteropathy (Lawsonia intracellularis infection). In: Journal of comparative pathology. Volume 129, Numbers 2-3, 2003 Aug-Oct, pp 179-185, ISSN 0021-9975 . PMID 12921724 .
- ^ AS Field, DJ Marriott, MC Hing: The Warthin-Starry stain in the diagnosis of small intestinal microsporidiosis in HIV-infected patients. In: Folia parasitologica. Volume 40, Number 4, 1993, pp. 261-266, ISSN 0015-5683 . PMID 7516907 .
- ↑ A. Slodkowicz-Kowalska: [Laboratory diagnostics of human microsporidiosis]. In: Wiadomo? Ci parazytologiczne. Volume 50, Number 4, 2004, pp. 679-689, ISSN 0043-5163 . PMID 16862802 .
- ^ HG Liu: Warthin-starry silver method showing particulate matter in macrophage. In: Biomedical and environmental sciences: BES. Volume 21, Number 1, February 2008, pp. 85-89, ISSN 0895-3988 . doi: 10.1016 / S0895-3988 (08) 60011-2 . PMID 18478983 .
- ↑ JG Brady et al .: Detection of mycobacterial infections using the Dieterle stain . In: Pediatr Dev Pathol . tape 1 , no. 4 , p. 309-313 , PMID 10463293 .
- ↑ PA Jones: A glutaraldehyde-dichromate-silver sequence for differentiating norepinephrine cells from epinephrine cells in neonatal and adult rats-paraffin sections. In: Stain technology. Volume 42, Number 1, January 1967, pp. 1-6, ISSN 0038-9153 . PMID 4166282 .
- ↑ LF Bonewald, SE Harris, J. Rosser, MR Dallas, SL Dallas, NP Camacho, B. Boyan, A. Boskey: von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. In: Calcified tissue international. Volume 72, Number 5, May 2003, pp. 537-547, ISSN 0171-967X . doi: 10.1007 / s00223-002-1057-y . PMID 12724828 .
- ↑ L. Kerenyi, F. Gallyas: About problems of the quantitative evaluation of the agar electrophoretograms silvered with physical development . In: Clin. Chim. Acta . tape 47 , no. 3 , 1973, p. 425-436 , doi : 10.1016 / 0009-8981 (73) 90276-3 , PMID 4744834 .
- ↑ CR Merril, RC Switzer, ML Van Keuren: Trace polypeptides in cellular extracts and human body fluids detected by two-dimensional electrophoresis and a highly sensitive silver stain. In: Proc Natl Acad Sci USA . 76 (9), 1979, pp. 4335-4339. PMID 92027 .
- ↑ RC Switzer, CR Merril, S. Shifrin: A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels . In: Anal Biochem . tape 98 , no. 1 , September 1979, p. 231-237 , doi : 10.1016 / 0003-2697 (79) 90732-2 , PMID 94518 .
- ↑ T. Rabilloud et al .: Improvement and simplification of low-background silver staining of proteins by using sodium dithionite. In: Electrophoresis. 9 (6), 1998, pp. 288-291. PMID 2466660 .
- ↑ T. Rabilloud: A comparison between low background silver diammine silver nitrate and protein stains. In: Electrophoresis. 13, 1992, pp. 429-439. PMID 1425556 .
- ↑ C. Lelong, M. Chevallet, S. Luche, T. Rabilloud: Silver staining of proteins in 2DE gels. In: Methods Mol Biol. 519, 2009, pp. 339-350. PMID 19381593 .
- ↑ H. Blum, H. Beier, HJ Gross: Improved silver staining of plant protein, RNA & DNA in PAA gels. In: Electrophoresis. 8, 1987, pp. 93-99.
- ↑ G. Dubray, G. Bezard: A highly sensitive periodic acid-silver stain for 1,2-diol groups of glycoproteins and polysaccharides in polyacrylamide gels. In: Anal. Biochem. . 119 (2), 1982, pp. 325-329. PMID 6176144 .
- ↑ E. Hempelmann, M. Schulze, O. Götze: Free SH groups are important for the polychromatic staining of proteins with silver nitrate . In: V. Neuhof (Ed.): Electrophoresis . Verlag Chemie, Weinheim 1984, p. 328-330 .
- ↑ D. Ochs: Protein contaminants of sodium dodecyl sulfate-polyacrylamide gels. In: Analytical biochemistry. Volume 135, Number 2, December 1983, pp. 470-474, PMID 6197906 .