Formose reaction
The formose reaction is a chemical reaction that produces a mixture of sugars ; the product is also called formose . The formose reaction is the self-condensation of formaldehyde on basic catalysts such as the oxides and hydroxides of calcium , barium , thallium and lead , but not of sodium , in the presence of CH-active compounds. It is believed that the formose reaction was a key reaction in the formation of the first biomolecules from the primordial soup and thus represents the basis for the origin of life.
They create central metabolic biomolecules such as glyceraldehyde , pentoses and hexoses from the simple C1 component formaldehyde. The pentoses are the basis of RNA , the carrier of genetic information in the prebiotic phase of evolution ( Origin of Live ). The reaction was discovered by Alexander Butlerow in 1861 and its complex mechanism has been studied over many generations of chemists.
reaction
The crucial point in this reaction is the first CC linkage. Formaldehyde cannot react with itself without catalysis because one electrophile does not react with another electrophile and there is only one carbon atom in formaldehyde. In a pure, alkaline formalin solution, normally only the Cannizzaro reaction takes place, which produces methanol and formic acid (Fig. 1).
For a CC linkage, one of the two carbon atoms is reversed , at least in the meantime . This is done in an elegant way in the formose cycle (Fig. 2).
If an alkaline formalin solution is inoculated with a small amount of an α-hydroxycarbonyl compound such as glycolaldehyde , an autocatalytic process develops within a short time , which steers the reaction in a different direction. Glyceraldehyde is produced from glycolaldehyde by aldol addition , which is converted into dihydroxyacetone via the Lobry-de-Bruyn-Alberda-van-Ekenstein rearrangement . This shift in the carbonyl group is essential for the further structure of the carbon chain, because it reverses the polarity of the reactivity pattern. While in glyceraldehyde was electrophilic on C1 and nucleophilic on C2, in dihydroxyacetone C2 becomes electrophilic and C1 or C3, which is formally derived from the previously electrophilic formaldehyde, becomes nucleophilic. A further aldol addition and thus a further chain extension can now take place there. Another van-Ekenstein rearrangement pushes back the reactivity pattern so that two moles of glycol are formed by retroaldol cleavage , which flow back into the autocatalytic cycle.
After the first cycle, the amount of glycolaldehyde doubles, after the second it is quadrupled, after the 10th it is a thousand times, after the 20th cycle the factor is a million, after the 30th it is a billion, etc. (Fig. 3)
The lower sugars glycolaldehyde and glyceraldehyde can in turn form C5 or C6 sugars via aldol condensations. As soon as the sugars have reached the size of 5 or 6 carbon atoms, they can form cyclic hemiacetals and are thus largely eliminated from the process as carbonyl compounds , so that no higher condensation products arise and thus the pentoses and hexoses, which are widespread in nature, are the stable end products of Reaction are.
Technically, this reaction can be used to manufacture artificial sugars and, after hydrogenolysis, to manufacture glycols (ethylene glycol, propylene glycol).
Selective forms of the formose reaction
Using vitamin B1- analogous umpolung catalysts based on thiazolium ylides , triazolium ylides and perimidinium ylides , the formose reaction can be selectively directed to glycolaldehyde or dihydroxyacetone.
| Catalytic polarity reversal | ||
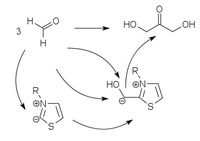
|
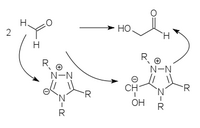
|

|
| Thiazolium catalysis | Triazolium catalysis | Perimidinium catalysis |
Technical importance of the formose reaction
In view of the limited petroleum reserves and the almost unlimited stocks of C1 sources (methane hydrate from the deep sea, coal, biogas), research in the field of the formose reaction in the chemical industry has been intensified since the 1990s because this is the only selective build-up reaction C1 basis and they enable a long-term conversion of the raw material basis.
Web links
- Albert Gossauer: Structure and reactivity of biomolecules , p. 330 ( limited preview in Google book search).
Individual evidence
- ^ A b Ronald Breslow : On the Mechanism of the Formose Reaction. In: Tetrahedron Letters , Vol. 1, No. 21, 1959, pp. 22-26 ( doi : 10.1016 / S0040-4039 (01) 99487-0 ).
- ^ A b Albert Eschenmoser : The search for the chemistry of life's origin. In: Tetrahedron , Vol. 63, 2007, pp. 12821-12844 ( doi : 10.1016 / j.tet.2007.10.012 ).
- ↑ Alexander Butlerov: Formation of a sugar-like substance through synthesis . In: Friedrich Wöhler, Justus Liebig, Hermann Kopp (eds.): Justus Liebig's annals of chemistry . tape 120 , no. 3 . CF Winter'sche Verlagshandlung, January 1861, ISSN 1099-0690 , p. 295–298 , doi : 10.1002 / jlac.18611200308 ( online on the pages of the Bayerische Staatsbibliothek BSB ).
- ↑ a b DE4023255 Process for the preparation of glycols, in particular propylene glycol / EP 0468320 Process for the preparation of glycols from formaldehyde.
- ↑ DE000004122669A1 (1991) Process for the production of dihydroxyacetone.
- ↑ DE000004212264A1 (1992) Process for the catalytic production of condensation products of formaldehyde.
- ↑ DE000004230466A1 (1992) Process for the catalytic production of condensation products of formaldehyde.
- ↑ DE000019536403A1 (1995) Perimidinium salts, their production and use.
- ^ J. Henrique Teles, Johann-Peter Melder, Klaus Ebel, Regina Schneider, Eugen Gehrer, Wolfgang Harder, Stefan Brode, Dieter Enders, Klaus Breuer, Gerhard Raabe: The Chemistry of Stable Carbenes. Part 2. Benzoin-type condensations of formaldehyde catalyzed by stable carbenes. In: Helvetica Chimica Acta , Vol. 79, No. 1, 1996, pp. 61-83 ( doi : 10.1002 / hlca.19960790108 ).
- ^ Gerhard Habermehl, Peter E. Hammann; Hans C. Krebs: Natural Products Chemistry. An introduction. 3rd, revised and expanded edition. Springer, Berlin et al. 2008, ISBN 978-3-540-73732-2 , p. 346 ( limited preview in the Google book search).


