Methyl acetate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Methyl acetate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 3 H 6 O 2 | ||||||||||||||||||
| Brief description |
colorless liquid with a fruity odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 74.08 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.93 g cm −3 |
||||||||||||||||||
| Melting point |
−99 ° C |
||||||||||||||||||
| boiling point |
57 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
easily in water (240–250 g · l −1 at 20 ° C) |
||||||||||||||||||
| Refractive index |
1.3614 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
|
||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−445.9 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Acetic acid methyl ester (according to IUPAC nomenclature: methyl acetate , systematically referred to as methyl ethanoate ) is an organic-chemical compound from the group of carboxylic acid esters .
Extraction and presentation
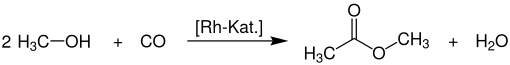
In industry, a large amount of methyl acetate is produced as a by-product in the production of acetic acid by the carbonylation of methanol .
This process is the Monsanto or further developed Cativa process for the large-scale production of acetic acid by carbonylation of methanol using rhodium or iridium complex catalysis .
Methyl acetate can also be produced by the direct acid-catalyzed esterification of acetic acid with methanol with reactive distillation.
In addition to mineral acids such as sulfuric or p -toluenesulphonic acid, acidic ion exchange resins or zeolites have recently been used as catalysts . The latter enable a simplified work-up of the product and elegant recovery of the catalyst. Finally, methyl acetate is obtained from the methanol / methyl acetate azeotrope by azeotropic distillation .
properties
Methyl acetate is a colorless liquid that boils at 57 ° C under normal pressure . According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in kPa, T in K) with A = 7.06524, B = 57.630 and C = 219.726. The critical values are 506.8 K for the critical temperature, 4688.8 kPa for the critical pressure and 228 · 10 −6 m 3 · mol −1 for the critical volume. The compound is readily soluble in water at 240 to 250 g / l at 20 ° C.
use
Methyl acetate is mainly used as a solvent for cellulose nitrate , cellulose acetate and others. Furthermore, it is permitted as an extraction agent to a limited extent. In addition, due to its volatility , methyl acetate is also used in the processing industry as a solvent for fast-drying paints . It is also used as a vaporizer for softening stiff caps and for the production of adhesives based on polyvinyl acetate and celluloid adhesives from film waste. Methyl acetate is also used in a wide variety of fields in the manufacture of dyes and in the perfume industry . The use in biodiesel production is also being researched in more recent work .
safety instructions
The vapors of methyl acetate can form explosive mixtures with air . Methyl acetate is mainly absorbed through the respiratory tract . Through this it is well absorbed , especially in the lungs and the digestive tract . Acute irritation to the eyes and respiratory tract occurs . A central nervous system disorder was also found. Chronic methyl acetate can cause skin damage through contact with the liquid . At high exposure there is a risk of damage to the optic nerve . A reproductive toxicity , mutagenicity or carcinogenicity has not yet been demonstrated or adequate results are not available. Methyl acetate has a lower explosion limit of approx. 3.1% by volume at 95 g · m −3 and an upper explosion limit of approx. 16.0% by volume at 495 g · m −3 . A correlation of the upper explosion limit with the vapor pressure function results in a lower explosion point of −19 ° C. The maximum explosion pressure is 9.6 bar. The ignition temperature is approx. 505 ° C. The substance therefore falls into temperature class T1 and explosion group IIA. The limit gap width was determined to be 0.97 mm at 50 ° C. With a flash point of −13 ° C, methyl acetate is considered highly flammable.
Individual evidence
- ↑ a b c d e f g h i j k l m n o Entry on methyl acetate in the GESTIS substance database of the IFA , accessed on January 17, 2019(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-334.
- ↑ Entry on Methyl acetate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 79-20-9 or methyl acetate ), accessed on September 14, 2019.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-24.
- ↑ a b c d Carole Le Berre, Philippe Serp, Philippe Kalck, G. Paull Torrence: Acetic Acid. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley ‐ VCH Verlag GmbH & Co. KGaA., March 26, 2014, p. 25, doi : 10.1002 / 14356007.a01_045.pub3 (section “Methyl acetate”).
- ↑ Boublik, T .; Fried, V .; Hala, E .: The Vapor Pressures of Pure Substances Elsevier 1975.
- ↑ a b Kapoor, S .; Gill, BK; Rattan, VK: Isobaric Vapor-Liquid Equilibrium of Binary Mixture of Methyl Acetate with Isopropylbenzene at 97.3 kPa in Int. J. Chem. Mol. Eng. 2 (2008) 281-284.
- ↑ Reid, RC; Prausnitz, JM; Poling, BE: The properties of Gases and Liquids, 4th ed. McGrawl-Hill, New York 1985.
- ↑ Entry on methyl acetate. In: Römpp Online . Georg Thieme Verlag, accessed on January 17, 2019.
- ↑ G. Doná, L. Cardozo-Filho, C. Silva, F. Castilhos: biodiesel production using super critical methyl acetate in a tubular packed bed reactor. In: Fuel Processing Technology . Volume 106, 2013, pp. 605-610, doi : 10.1016 / j.fuproc.2012.09.047 .