Henry reaction
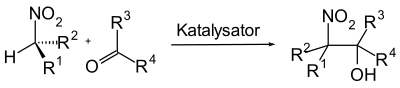
The Henry reaction , also known as the Kamlet reaction or the nitroaldol reaction , is a name reaction in organic chemistry . Characteristic of this reaction is the coupling of an α-carbon atom of an aliphatic nitro compound with a carbonyl compound to form a β-nitro alcohol with the formation of a C – C bond.
The Henry reaction is usually used as an intermediate reaction step in the synthesis of complex organic molecules. It is carried out under relatively mild reaction conditions and, with the formation of β-nitroalcohol, offers a functionalized starting compound for further transformations such as oxidations , reductions or the Nef reaction .
Because of the mild reaction conditions, unstable protective groups and functional groups are also retained.
mechanism
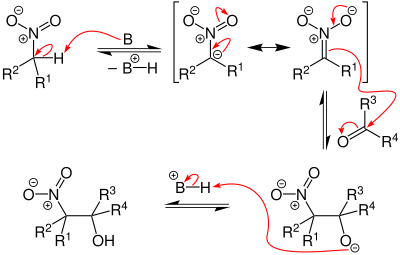
The reaction mechanism of the Henry reaction is very similar to that of the aldol reaction , which is why it is often referred to as the nitroaldol reaction. The difference to the aldol reaction is that in the Henry reaction the C – H acidic carbonyl compound was replaced by a nitro compound.
Aliphatic nitro compounds that carry an α-hydrogen atom are very C – H acidic and can be deprotonated by strong bases. A nitronate ion is formed in the process. The nitronate ion can now attack the carbonyl compound on the carbonyl carbon atom, whereby the anion of the β-nitro alcohol is formed. Subsequent protonation produces the β-nitro alcohol.
Stereochemistry
In the Henry reaction, a prochiral aldehyde or ketone is usually converted into a chiral compound by reacting with the nitronate ion. An exception is the use of symmetrical carbonyl compounds such as acetone , as these compounds are not prochiral and therefore no stereocenter is formed. The same applies to the nitro component. When using nitromethane, no new stereocenter is created either.
A newly formed stereocenter results in two possible enantiomers. With two newly formed stereocenters, on the other hand, four possible compounds are obtained - two pairs of enantiomers and two pairs of diastereomers. A distinction is made between the threo and erythro form (derived from threose and erythrosis ) for the pairs of diastereomers . If both substituents are on one side in the Fischer projection , this is the erythro form; if both substituents are on the opposite side, it is the threo form.
If all the compounds involved in the reaction are achiral or are present as a mixture of stereoisomers, then in the Henry reaction, as is generally the case with such reactions, no stereoisomer is formed preferentially, and that means a uniform mixture of all possible enantiomers is always obtained. The situation is different with the diastereomer pairs, which, due to steric effects, can definitely have a preference even in the absence of an enanatiomer-enriched compound, which is why the ratio threo : erythro is often determined here in order to make statements about the selectivity of a reaction.
One has a rather complicated case when one or both substrates already contain stereocenters (e.g. sugars or aldehydes which are derived from amino acids ), since more combinations of enantiomer and diastereomer pairs are conceivable here, and the stereocenters as well Influence selectivities yourself.
Catalysts
The Henry reaction can be catalyzed by a variety of catalysts and reaction conditions. Organic bases, inorganic bases, quaternary ammonium salts, protic and non-protic solvents, and solvent-free conditions have been used successfully. This allows the reaction conditions to be adapted to the substrates, which z. B. protecting groups, solubilities and lability of functional groups.
The first catalysts used in the Henry reaction were both alkoxides and hydroxides in alcoholic or aqueous solvents. A later development was the use of 1,1,3,3-tetramethylguanidine (TMG) as a catalyst, which has been used several times.
Based on this, bicyclic guanidines and even guanidines bound to polymeric resins were used.
Verkade then developed proazaphosphatanes as effective catalysts for the Henry reaction in the reaction of nitroalkanes with aldehydes and ketones.
When ketones are used as carbonyl components, the condensation of the ketones with one another ( aldol condensation ) is the main side reaction. This side reaction can be effectively suppressed by using the proazaphosphatanes. Magnesium sulfate is used as the Lewis acid in the Vekaden reagent system for optimal conditions .
Numerous nitroaldol reactions between aromatic and aliphatic aldehydes and nitromethane , nitroethane and 1-nitropropane have also been published with lithium aluminum hydride as the catalyst .
R. Ballini presented the Amberlyst A-21 ion exchanger under solvent-free conditions in the production of nitrodiols by reacting aldehydes with 4-nitro-butanol . The application of the phase transfer catalyst cetyltrimethylammonium chloride in water and in the presence of sodium hydroxide originates from the same working group .
MJ Shibasaki published catalysts based on rare earths. Samarium trihexamethylsilazide [Sm (HDMS) 3 ], which is easy to prepare from samarium (III) chloride (SmCl 3 ) and sodium hexamethyldisilazide (NaHMDS), deserves a special mention here because it provides the basis for the chiral catalysts .
Asymmetric Catalysis
The aim of asymmetric catalysis is to enable the highest possible proportion of an enantiomer to be synthesized. Since most active ingredients have different effects in vivo , it is of great interest for large-scale synthesis to have synthetic methods that are as selective as possible at hand.
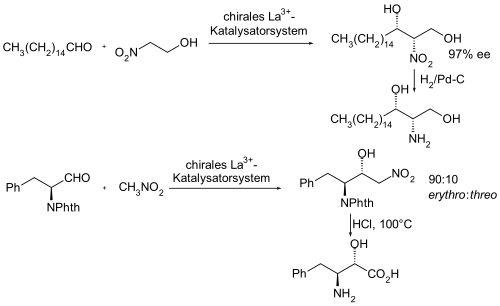
The first catalytic asymmetric Henry reaction was published in 1992 by H. Shibasaki et al. published. The experience gained with rare earth catalysts was used and initially lanthanum was used as the metal. Enantiomerically pure β-binaphthol was used as a chiral auxiliary or ligand . In a later publication, the synthesis of the catalyst system was simplified and instead of the lanthanum source, trilanthan heptate tert- butoxide, the easier-to-use lanthanum (III) chloride was used as the lanthanum source . The enantiomeric excess achieved was stated to be 79-91%.
Medium to high enantiomeric excesses (70–95%) were also achieved when samarium was used as the metal, and binaphthol as a chiral ligand was also used here.
Based on previous experience with binaphthol as a chiral ligand and the guanidines as achiral leagues, it made sense to continue working on this basis to optimize the ligands. Various chiral guanidines were used in this work.
In the following, two asymmetric syntheses using an asymmetrically catalyzed Henry reaction as a partial synthesis of pharmacologically interesting compounds are shown as examples.
Variations of the Henry reaction
Nitronate condensation
If a nitroalkane is reacted with n -butyllithium , a lithioalkylnitronate is obtained which, in the presence of isopropyloxytitanium trichloride , can be reacted with many aldehydes under very mild conditions (THF, −78 ° C) to give the corresponding β-aminoalkanols. The in-situ formation of a dichloroisopropylnitronate supports the high diastereoselectivity of the nitroalkanol products ( erythro : threo = 7: 1).
A variant comes from Seebach and Eyer in which a dilithiated nitronate is used as the active nitronate species in a Henry reaction. The potential of this method could be demonstrated in a number of reactants such as benzyl halides or dimethyl carbonate as carbonyl components. An example is a tetrahydropyranyl -protected nitroethanol , which is mixed with a twofold excess of n -butyllithium in THF / HMPT in order to obtain the nitronate. The nitronate is a unintramolecular chelated nitronate dianion. This was reacted with a number of aldehydes and after acidic treatment (Amberlyst) the dihydroxynitroalkanes are obtained. It was found that the reaction products are preferably those of the threo series. After the THP protective group had been split off, the pure diastereomers could be obtained by crystallization.
Retro henry reaction
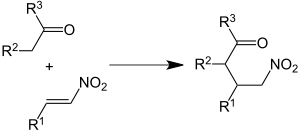
In analogy to the related aldol reaction, the Henry reaction is a reversible reaction. This can be exploited if one prepares a β-hydroxnitro compound in a way other than a Henry reaction and then subjects it to a retro-Henry reaction. For this purpose, β-nitroketones are particularly suitable, which are converted into such a β-hydroxnitro compound by reaction with Grignard reagents with CC linkage.
Michael-Henry reaction
The Michael reaction is a vinylogous aldol reaction. In analogy to this, there is also a Michael-Henry reaction (nitro-Michael reaction). Here the α-β-unsaturated nitro group reacts like the Michael acceptor in the Michael reaction.
Intramolecular Henry reaction
Like many other organic reactions, the Henry reaction can also be carried out intramolecularly. The nitro component and carbonyl component are present within the same molecule and the Henry reaction leads to a ring-closing reaction. As a rule, either the nitro component or the carbonyl component are prepared in a preliminary step and this compound is then subjected to a cyclization reaction. Here is an example from natural product synthesis. The carbonyl component is obtained from an ester by Dibal reduction (DIBAL-H) to form an aldehyde. The cyclization is then initiated by aluminum oxide .
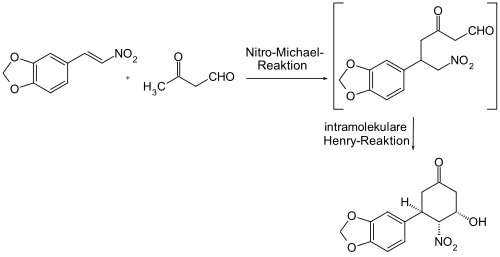
A similar scaffold can be built by a combination of the Henry-Michael reaction and the Henry reaction.
In a variant of the intramolecular Henry reaction, the active species is first built up intermolecularly through a Henry reaction from carbonyl components and nitro components. For this purpose, a dicarbonyl compound is required, which is accessible by oxidation of a diol or from olefins by oxidation to glycol and subsequent periodate cleavage or directly by ozonolysis . The reaction with a nitroalkane leads to a compound which contains a nitro group and a carbonyl group in the molecule and can be subjected to the intramolecular Henry reaction.
literature
- Louis Henry: Formation synthétique d'alcools nitrés. In: Comptes rendus hebdomadaires des séances de l'Académie des sciences . 120, 1895, pp. 1265-1268 ( digitized on Gallica ). [1]
- Patent US2151517 : Preparation of arylnitroalkanols. Published on March 21, 1939 , inventor: J. Kamlet (cf. Chem. Abstr. Vol. 33, No. 9, 1939, p. 5003).
- HB Hass, Elizabeth F. Riley: The Nitroparaffins. In: Chemical Reviews. 32, No. 3, 1943, pp. 373-430, doi: 10.1021 / cr60103a003 .
- Dieter Seebach, Ernest W. Colvin, Friedrich Lehr, Thomas Weller: Nitroliphatic Compounds — Ideal Intermediates in Organic-synthesis. In: Chimia. 33, No. 1, 1979, pp. 1-18.
- Goffredo Rosini: The Henry (Nitroaldol) Reaction . In: Barry M. Trost (Ed.): Comprehensive Organic Synthesis . Vol. 2 Additions to CX π-Bonds, Part 2, Pergamon, Oxford 1991, pp. 321-340 doi: 10.1016 / B978-0-08-052349-1.00032-9 .
- Frederick A. Luzzio: The Henry reaction: recent examples. In: Tetrahedron. 57, No. 6, 2001, pp. 915-945, doi : 10.1016 / S0040-4020 (00) 00965-0 .
Individual evidence
- ↑ Mundy, Bradford P., Michael G. Ellerd, and Frank G. Favaloro Jr. Name reactions and reagents in organic synthesis. John Wiley & Sons, 2005. pp. 300-301
- ↑ Louis Frederick Fieser , Mary Fieser, Tse-Lok Ho: Reagents for Organic Synthesis . Vol. 1, Wiley, New York 1967, ISBN 0-471-25875-X , p. 739.
- ^ Angus C. Forsyth, R. Michael Paton, Ian Watt: Highly selective base-catalysed additions of nitromethane to levoglucosenone. In: Tetrahedron Letters. 30, No. 8, 1989, pp. 993-996, doi : 10.1016 / S0040-4039 (00) 95299-7 .
- ^ Richard W. Fitch, Frederick A. Luzzio: The aluminum amalgam reduction of 2-nitroalkanols promoted by ultrasound. In: Tetrahedron Letters. 35, No. 33, 1994, pp. 6013-6016, doi : 10.1016 / 0040-4039 (94) 88062-X .
- ↑ Daniele Simoni, Francesco Paolo Invidiata, Stefano Manfredini, Roberto Ferroni, Ilaria Lampronti, Marinella Roberti, Gian Piero Pollini: Facile synthesis of 2-nitroalkanols by tetramethylguanidine (TMG) -catalyzed addition of primary nitroalkanes to aldehydes and alicyclic ketones. In: Tetrahedron Letters. 38, No. 15, 1997, pp. 2749-2752, doi : 10.1016 / S0040-4039 (97) 00461-9 .
- ↑ Marco Bandini, Pier Giorgio Cozzi, Meri de Angelis, Achille Umani-Ronchi *: Zinc triflate – bis-oxazoline complexes as chiral catalysts: enantioselective reduction of α-alkoxy-ketones with catecholborane. In: Tetrahedron Letters. 41, No. 10, 2000, pp. 1601-1605, doi : 10.1016 / S0040-4039 (99) 02339-4 .
- ↑ Philip B. Kisanga, John G. Verkade: P (RNCH 2 CH 2 ) 3 N: An Efficient Promoter for the Nitroaldol (Henry) Reaction In: The Journal of Organic Chemistry. 64, No. 12, 1999, pp. 4298-4303, doi : 10.1021 / jo9818733 .
- ↑ YSO Won Youn, Yong Hae Kim: Facile Synthesis of 2-Mediated Nitroalkanols with LiAlH 4 as Catalyst. In: Synlett. 2000, No. 6, 2000, pp. 880-882, doi : 10.1055 / s-2000-6730 .
- ↑ Roberto Ballini, Giovanna Bosica: ... A New Stereoselective Synthesis of (E) - alpha, beta.-Unsaturated-.gamma.-dicarbonyl compounds by the Henry reaction. In: The Journal of Organic Chemistry. 59, No. 18, 1994, pp. 5466-5467, doi : 10.1021 / jo00097a061 .
- ↑ Roberto Ballini, Giovanna Bosica: Nitroaldol Reaction in Aqueous Media: An Important Improvement of the Henry reaction. In: The Journal of Organic Chemistry. 62, No. 2, 1997, pp. 425-427, doi : 10.1021 / jo961201h .
- ↑ Hiroaki Sasai, Shigeru Arai, Masakatsu Shibasaki: Catalytic Aldol Reaction with Sm (HMDS) 3 and Its Application for the Introduction of a Carbon-Carbon Triple Bond at C-13 in Prostaglandin Synthesis. In: The Journal of Organic Chemistry. 59, No. 9, 1994, pp. 2661-2664, doi : 10.1021 / jo00088a068 .
- ↑ Hiroaki Sasai, Takeyuki Suzuki, Shigeru Arai, Takayoshi Arai, Masakatsu Shibasaki: Basic character of rare earth metal alkoxides. Utilization in catalytic carbon-carbon bond-forming reactions and catalytic asymmetric nitroaldol reactions. In: Journal of the American Chemical Society. 114, No. 11, 1992, pp. 4418-4420, doi : 10.1021 / ja00037a068 .
- ↑ Hiroaki Sasai, Takeyuki Suzuki, Noriie Itoh, Masakatsu Shibasaki: Catalytic asymmetric nitroaldol reactions. A new practical method for the preparation of the optically active lanthanum complex. In: Tetrahedron Letters. 34, No. 5, 1993, pp. 851-854, doi : 10.1016 / 0040-4039 (93) 89030-T .
- ↑ Katsuhiko Iseki, Satoshi Oishi, Hiroaki Sasai, Masakatsu Shibasaki: Catalytic asymmetric nitroaldol reaction of α, α-difluoro aldehydes mediated by rare earth-lithium-BINOL complexes. In: Tetrahedron Letters. 37, No. 50, 1996, pp. 9081-9084, doi : 10.1016 / S0040-4039 (96) 02090-4 .
- ↑ Rafael Chinchilla, Carmen Nájera, Pablo Sánchez-Agulló: Enantiomerically pure guanidine-catalysed asymmetric nitroaldol reaction. In: Tetrahedron: Asymmetry . 5, No. 7, 1994, pp. 1393-1402, doi : 10.1016 / 0957-4166 (94) 80183-5 .
- ↑ Anthony P. Davis, Kevin J. Dempsey: Synthesis and investigation of a hindered, chiral, bicyclic guanidine. In: Tetrahedron: Asymmetry. 6, No. 11, 1995, pp. 2829-2840, doi : 10.1016 / 0957-4166 (95) 00374-X .
- ↑ Hiroaki Sasai, Noriie Itoh, Takeyuki Suzuki, Masakatsu Shibasaki: Catalytic asymmetric nitroaldol reaction: An efficient synthesis of (S) propranolol using the lanthanum binaphthol complex. In: Tetrahedron Letters. 34, No. 5, 1993, pp. 855-858, doi : 10.1016 / 0040-4039 (93) 89031-K .
- ↑ Hiroaki Sasai, Won-Sup Kim, Takeyuki Suzuki, Masakatsu Shibasaki, Masaru Mitsuda, Junzo Hasegawa, Takehisa Ohashi: Diastereoselective catalytic asymmetric nitroaldol reaction utilizing rare earth-Li- (R) -BINOL complex. A highly efficient synthesis of norstatine. In: Tetrahedron Letters. 35, No. 33, 1994, pp. 6123-6126, doi : 10.1016 / 0040-4039 (94) 88093-X .
- ↑ Anthony GM Barrett, Chantal Robyr, Christopher D. Spilling: Dichloroisopropoxytitanium nitronates as reagents for stereoselective Henry reactions. In: The Journal of Organic Chemistry. 54, No. 6, 1989, pp. 1233-1234, doi : 10.1021 / jo00267a001 .
- ↑ Martin Eyer, Dieter Seebach: l-2-Nitro-1,3-alkanediols by stereoselective addition of nitroethanol to aldehydes. On the asymmetric electrophilic addition to double bonds. In: Journal of the American Chemical Society. 107, No. 12, 1985, pp. 3601-3606, doi : 10.1021 / ja00298a033 .
- ↑ Maitreyee (Sarma) Bezbarua, Anil K. Saikia, Nabin C. Barua, Dipak Kalita, Anil C. Ghosh: A Short Enantioselective Formal Synthesis of Methyl (S) - (-) - 6,8-Dihydroxyoctanoate: A Key Intermediate for the Synthesis of R - (+) - α-Lipoic Acid. In: Synthesis. 1996, No. 11, 1996, pp. 1289-1290, doi : 10.1055 / s-1996-4380 .
- ↑ Anil K. Saikia, Monoj J. Hazarika, Nabin C. Barua, Maitreyee Sarma Bezbarua, Ram P. Sharma, Anil C. Ghosh: Direct Synthesis of Keto Nitroaliphatics via Retro-Henry Reaction of Cyclic 2-Nitroalcohols by Anhydrous Copper Sulfate Adsorbed on silica gel. A Short Synthesis of (±) -Phoracantholide I. In: Synthesis. 1996, No. 8, 1996, pp. 981-985, doi : 10.1055 / s-1996-4318 .
- ↑ James McNulty, Ruowei Mo: Diastereoselective intramolecular nitroaldol entry to lycoricidine alkaloids. In: Chemical Communications. , No. 8, 1998, pp. 933-934, doi : 10.1039 / A800097B .
- ↑ Thomas Weller, Dieter Seebach: Synthesis of (±) -1-deoxy-2-lycorinone and of a possible trans-dihydro-lycoricidine precursor. In: Tetrahedron Letters. 23, No. 9, 1982, pp. 935-938, doi : 10.1016 / S0040-4039 (00) 86987-7 .
- ↑ M. Eberle, M. Missbach, D. Seebach: Enantioselective Saponification with Pig Liver Esterase (PLE): (1S, 2S, 3R) -3-Hydroxy-2-nitrocyclohexyl Acetate In: Organic Syntheses . 69, 1990, p. 19, doi : 10.15227 / orgsyn.069.0019 ; Coll. Vol. 8, 1993, p. 332 ( PDF ).