Nef reaction
The Nef reaction is a name reaction in organic chemistry . The reaction was named after the American chemist John Ulric Nef (1862–1915). In the Nef reaction, a primary or secondary nitroalkane is converted into the corresponding aldehyde or ketone and nitrous oxide (N 2 O) under acid catalysis .
John Ulric Nef discovered the reaction when he treated the sodium salt of nitroethane with sulfuric acid in 1894 and obtained nitrous oxide with a yield of 85 to 89% and acetaldehyde with 70% . A year earlier, however, Konovalov had published a similar reaction. He converted the potassium salt of 1-phenylnitroethane with sulfuric acid to form acetophenone .
Reaction mechanism
The nitroalkane 1 is deprotonated. The resulting salt is then protonated to the nitronate 4 and a further protonation to the iminium ion 5 takes place. The nucleophilic attack of water on 5 and a deprotonation now results in the 1-nitrosoalkanol 8 , which is assumed to be responsible for the blue color of the reaction solution . Rearrangement and cleavage result in the end product 9 and hypositrous acid . This is split off and breaks down into nitrous oxide and water.
The reaction requires an acidic hydrogen in the α position (i.e. on the carbon atom to which the nitro group is bound) and can therefore not be carried out with tertiary nitro compounds.
Application examples
In carbohydrate chemistry , the Nef reaction is sometimes used to chain an aldose. One example is the isotope labeling of 14 C-D- mannose and 14 C-D- glucose based on D- arabinose and 14 C- nitromethane .
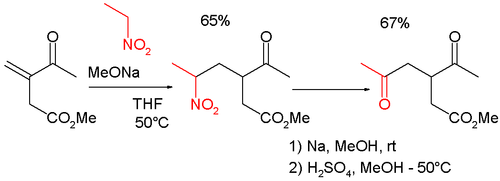
Synthesis of γ-ketocarbonyl compounds
The reaction is also sometimes used in combination with the Michael reaction to synthesize γ- ketocarbonyl compounds:
Individual evidence
- ^ Wayland E. Noland: The NEF Reaction . In: Chemical Reviews . tape 55 , no. 1 , February 1955, p. 137-155 , doi : 10.1021 / cr50001a003 .
- ↑ Harold W. Pinnick: The NEF Reaction . In: Organic Reactions . tape 38 , no. 1 , 1990, p. 655-792 , doi : 10.1021 / cr50001a003 .
- ↑ DS Grierson, H.-P. Husson: Polonovski and Pummerer-type Reactions and the Nef Reaction . In: Barry M. Trost, Ian Fleming, Ekkehard Winterfeldt (eds.): Comprehensive Organic Synthesis . tape 6 . Pergamon Press, Oxford, New York, Seoul, Tokyo 1991, ISBN 978-0-08-040597-1 , pp. 938-944 .
- ↑ JU Nef: About the constitution of the salts of the nitroparaffins . In: Justus Liebig's Annals of Chemistry . tape 280 , no. 2-3 , 1894, pp. 263-291 , doi : 10.1002 / jlac.18942800209 .
- ↑ M. Konowalow: About the effect of acids on salts of nitro compounds . In: Reports of the German Chemical Society . tape 29 , no. 2 , May 1896, p. 2193 , doi : 10.1002 / cber.189602902215 .
- ↑ Nour Lahmar, Taïcir Ben Ayed, Moncef Bellassoued, Hassen Amri: A convenient synthesis of γ-functionalized cyclopentenones . In: Beilstein Journal of Organic Chemistry . tape 1 , 2005, doi : 10.1186 / 1860-5397-1-11 .