Hypo-nitrous acid
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
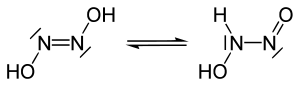
|
||||||||||
| Isomeric forms of hypositrous acid | ||||||||||
| General | ||||||||||
| Surname | Hypo-nitrous acid | |||||||||
| other names |
|
|||||||||
| Molecular formula | H 2 N 2 O 2 | |||||||||
| Brief description |
colorless platelets that are explosive when dry |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 62.0 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| pK s value |
|
|||||||||
| solubility |
good in water and ethanol |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Hypo-nitrous acid is an oxygen acid of nitrogen . It forms colorless crystals that are explosive. These are soluble in ethanol and water.
Their salts are called hyponitrites , e.g. E.g .: potassium hyponitrite (K 2 N 2 O 2 ).
Manufacturing
trans -Hypositrous acid can be produced by reducing nitrous acid or sodium nitrite with nascent hydrogen (e.g. from sodium amalgam ).
The water-soluble sodium salt can be separated as yellow, insoluble silver hyponitrite with silver nitrate :
After separation, the trans -Hypositrous acid can be redissolved in ether with HCl gas:
properties
trans -Hypositrous acid is a colorless solid that is in the form of deliquescent crystal flakes and is a very weak acid. It is very explosive, fizzles out when rubbed with a glass rod and spontaneously decomposes even for no apparent reason and even when cooled to −6 ° C. It is very easily soluble in water and in ethanol , easily also in ether , benzene and trichloromethane , but difficult in ligroin .
Isomerism
There is a cis - and a more stable trans -Hyposalpetrige acid.
Only the salts of the cis form are known; the trans form can be isolated in substance.
Reactions
With alkali metals constituting hyponitrous acid salts , the hyponitrites :
The weakly acidic aqueous solution of the acid breaks down to nitrous oxide and water at room temperature :
The reaction is irreversible; Dinitrogen monoxide is therefore only formally anhydride of hypo- nitrous acid.
It is also suitable as a reaction partner for hetero Diels-Alder reactions .
Individual evidence
- ↑ a b Entry on hyposalpetretic acid. In: Römpp Online . Georg Thieme Verlag, accessed on November 10, 2014.
- ↑ a b c d e A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b G. Brauer (Ed.), Handbook of Preparative Inorganic Chemistry 2nd ed., Vol. 1, Academic Press 1963, pp. 492-6.
- ↑ E. Riedel, C. Janiak: Inorganic Chemistry . 8th edition. de Gruyter, 2011, ISBN 3-11-022566-2 , p. 488 .





