Glycolonitrile
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Glycolonitrile | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 2 H 3 N O | ||||||||||||||||||
| Brief description |
colorless oily liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 57.05 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.10 g cm −3 |
||||||||||||||||||
| Melting point |
<−72 ° C |
||||||||||||||||||
| boiling point | |||||||||||||||||||
| Vapor pressure |
63 mmHg (20 ° C) |
||||||||||||||||||
| solubility |
very soluble in water, ethanol and diethyl ether , insoluble in benzene |
||||||||||||||||||
| Refractive index |
1.4117 (19 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Glycolonitrile (hydroxyacetonitrile) is a very water-soluble α-hydroxynitrile and the simplest cyanohydrin , which is formed by the addition of hydrogen cyanide to formaldehyde .
Since glycolonitrile easily breaks down into the poisonous starting compounds and polymerizes in the alkaline, hydroxyacetonitrile is traded in acidic aqueous solutions (approx. 50 to 70%).
Manufacturing
The formation of glycolonitrile from formaldehyde and hydrogen cyanide was mentioned in the literature as early as 1890:
The likely existence of both reactants during the prebiotic phase of Earth's history suggests the spontaneous formation of glycolonitrile (and its derivatives) at an early stage in geochemistry .
Glycolonitrile is produced as the first stage of the biotransformation of acetonitrile by an NADPH -dependent cytochrome P450 monooxygenase in human and animal cells.
After a laboratory procedure can glycolonitrile by the reaction of equimolar amounts of potassium cyanide and formaldehyde in aqueous solution by addition of dilute sulfuric acid to the release of hydrogen cyanide yield after intensive extraction with diethyl ether followed by vacuum distillation to 76-80% are obtained. The laboratory procedure is based on an early publication in which this reaction was already described and the product glycolonitrile was obtained in 88% yield after distillation.
The instability of glycolonitrile and its tendency to spontaneously violent decomposition or polymerization under thermal stress and higher pH values are already indicated in the older literature, and the addition of acids is recommended for processing and storage.
In order to suppress the formation of by-products, equimolar amounts of purified formaldehyde as a 37% formalin solution and ammonia-free hydrogen cyanide are reacted at 30 ° C and pH 5.5 and the glycolonitrile obtained is stabilized to pH 2 by acidification.
In a continuous process, the reactants NH 3 and (HCN + HCHO) are reacted in a ratio of 3: 1 in a tube reactor at approx. 150 ° C. and a residence time of approx. 15 seconds, with an almost complete reaction to form glycolonitrile. Iminodiacetonitrile (IDAN) HN (CH 2 CN) 2 is obtained as a by-product due to the two-fold substitution of the hydrogen atoms in the ammonia .
Particularly pure (formaldehyde-free) glycolonitrile is obtained when the oligomers and polymers of formaldehyde present in the formalin solution are depolymerized by heating to 120 ° C. before the reaction with HCN. If the reactants are continuously metered in, the reaction gives glycolonitrile in yields of up to 95% and purities of up to 99.9% at 20 ° C.
The production of glycolonitrile by the oxidation of acetonitrile with oxygen in the vapor phase over metal catalysts is of no interest because of low selectivities and yields.
properties
Glycolonitrile is a clear, colorless, oily and poisonous liquid that easily breaks down into the poisonous starting materials formaldehyde and hydrogen cyanide and can polymerise explosively under the action of alkalis.
Applications
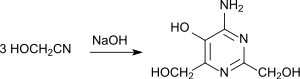
Glycolonitrile trimerizes in dilute aqueous solution at pH 8 and 0 ° C in 47% yield to give 4-amino-5-hydroxy-2,6-di (hydroxymethyl) pyrimidine:
The reaction with excess ammonia produces aminoacetonitrile ,
which is converted into the amino acid glycine by alkaline (in addition to glycine, other α-amino acids such as alanine or threonine ) or acid hydrolysis of the nitrile group .
Glycine can also be obtained directly from glycolonitrile by reacting with excess ammonia and carbon dioxide in good yield (approx. 85%) and purity (> 98%) with the formation of the corresponding hydantoin and subsequent cleavage into the amino acid.
or.
The iminodiacetonitrile (IDAN), which often occurs as a by-product of the glycolonitrile synthesis, can be selectively produced by reacting glycolonitrile with ammonia at pH 5 and 70 ° C in yields of around 80% by substituting two hydrogen atoms of the ammonia with two -CH 2 -CN groups produce.
The synthesis of nitrilotriacetonitrile (NTAN, by substituting all hydrogen atoms in ammonia with three -CH 2 -CN groups) according to the gross reaction equation
probably proceeds via intermediate glycolonitrile. This course of the reaction was postulated in an early publication.
Glycolic acid is obtained from glycolonitrile via a nitrilase-catalyzed enzymatic conversion to ammonium glycolate, which is obtained by means of electrodialysis , treatment with ion exchangers , reactive extraction, e.g. B. with long-chain (C 8 -C 10 ) trialkylamines, or esterification with methanol to give methyl glycolate and subsequent hydrolysis.
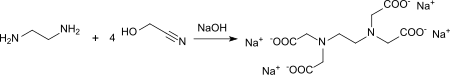
Glycolonitrile (4 mol) reacts with ethylenediamine (1 mol) in the presence of sodium hydroxide solution to form the tetrasodium salt of ethylenediaminetetraacetic acid (EDTA) - a frequently used complexing agent . Reaction conditions and yields are not given.
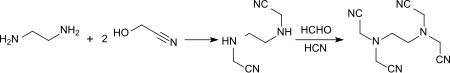
The reaction of glycolonitrile (2 mol) with ethylenediamine (1 mol) at 60 ° C. gives ethylenediamine diacetonitrile (EDDN) which, with equimolar amounts of HCN and HCHO at 60 ° C., gives ethylenediaminetetraacetonitrile (EDTN) in 74% yield.
The tetrasodium salt of ethylenediaminetetraacetic acid (EDTA) is formed analogously by hydrolysis of the tetranitrile with 40% sodium hydroxide solution .
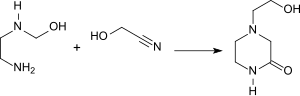
With ethylenediamine or substituted ethylenediamines, such as. B. 2- (2-Aminoethylamino) ethanol are formed by reaction with glycolonitrile at 100 ° C 2-piperazinones, which are suitable as an absorption medium for sulfur dioxide in gas scrubbers .
The relatively easily biodegradable (80% in 5 days) complexing agent 2-hydroxyethyliminodiacetic acid (HEIDA) can be obtained by reacting glycolonitrile twice with 2-aminoethanol .
Because of its instability, glycolonitrile is not isolated as an intermediate product in reactions with hydrogen cyanide and formaldehyde, but rather directly to the desired end product, e.g. B. Glycine, IDAN, NTAN or EDTN processed.
The oligomerization of glycolonitrile to aminooxazoles is discussed as a possible pathway for the formation of heterocycles in early geological history.
Individual evidence
- ↑ a b c d data sheet formaldehyde cyanohydrin at Cameo Chemicals.
- ↑ a b c d Lide, DR (ed.), CRC Handbook of Chemistry and Physics, 79st Edition, CRC Press LLC, Boca Raton, FL 2000, p. 3-10
- ↑ a b K. Polstorff, H. Meyer: On the action of potassium cyanide on formaldehyde . In: Ber. German chem. Ges. Band 45 , no. 2 , 1912, pp. 1905–1912 , doi : 10.1002 / cber.19120450263 .
- ↑ a b c Patent US2723979 : 4-Amino-5-hydroxy-2,6-di (hydroxymethyl) -pyrimidine and process of preparing same. Applied on August 2, 1954 , published November 15, 1955 , applicant: EI du Pont de Nemours and Company, inventor: DB Lake.
- ↑ a b Entry on glycolonitrile in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ^ Richard J. Lewis, Sr., Hazardous Chemicals Desk Reference, 6th Edition, John Wiley & Sons, Hoboken, NJ 2008, p. 749.
- ↑ Data sheet glycolic acid nitrile solution at Sigma-Aldrich , accessed on March 1, 2016 ( PDF ).
- ^ L. Henry: Sur le nitrile glycolique et la synthèse directe de l'acide glycolique . In: Comp. Rend. tape 110 , 1890, pp. 759-760 .
- ^ HJ Cleaves II: The prebiotic geochemistry of formaldehyde . In: Precambrian Res. Band 164 , 2008, p. 111–118 , doi : 10.1016 / j.precamres.2008.04.002 .
- ↑ International Program on Chemical Safety: Environmental Health Criteria 154. Acetonitrile . Ed .: World Health Organization . Geneva 1993 ( online ).
- ↑ R. Gaudry: Glycolonitrile . In: Org. Synth., Coll. Vol. Volume 3 , 1955, pp. 436 , doi : 10.15227 / orgsyn.027.0041 .
- ↑ Patent US2175805 : Stabilized organic nitrile and process of making. Applied on June 8, 1937 , published October 10, 1939 , applicant: EI du Pont de Nemours & Company, inventor: RA Jacobson.
- ↑ a b Patent US5187301 : Preparation of iminodiacetonitrile from glycolonitrile. Filed October 11, 1990 , published February 16, 1993 , Applicant: WR Grace & Co., Inventor: BA Cullen, BA Parker.
- ↑ Patent US5079380 : Adiabatic process for the preparation of glycolonitrile. Applied on May 23, 1990 , published January 7, 1992 , applicant: WR Grace & Co., inventor: JC Thunberg.
- ↑ Patent EP1833784B1 : Process for the synthesis of glycolonitrile. Filed December 21, 2005 , published October 20, 2010 , applicant: EI du Pont de Nemours and Company, inventor: T. Foo, A. Panova.
- ↑ Patent US4515732 : Conversion of acetonitrile to glycolonitrile and / or glycolamide. Filed May 29, 1984 , published May 7, 1985 , Applicant: The Standard Oil Company, Inventor: JF Brazdil, Jr., WA Marritt, MD Ward.
- ↑ DB Lake, TE Londergan: The trimerization of glycolonitrile . In: J. Org. Chem. Band 19 , no. 12 , 1954, pp. 2004-2007 , doi : 10.1021 / jo01377a018 .
- ↑ Patent DE677713 : Process for the production of aminonitriles. Registered on January 21, 1937 , published on June 8, 1939 , applicant: Mining Association for the Exploitation of Protective Rights of Coal Technology in Dortmund-Eving, inventor: W. Klempt.
- ^ RE Moser, CN Matthews: Hydrolysis of aminoacetonitrile: Peptide formation . In: Experientia . tape 24 , no. 7 , 1968, p. 658-659 , doi : 10.1007 / BF02138294 .
- ^ WK Anslow, H. King: Glycine [(A) (from Methyleneaminoacetonitrile)] . In: Org. Synth. tape 4 , 1925, pp. 31 , doi : 10.15227 / orgsyn.004.0031 .
- ↑ Patent EP0441588B1 : Process for preparing glycine. Applied on February 5, 1991 , published May 10, 1995 , Applicants: Mitsui Toatsu Chemicals, Inc., Inventors: K. Fujiwara, Y. Matsuu, N. Ueda, H. Kato, A. Hiai.
- ↑ Patent US5008428 : Integrated process for the production of aminoacetonitriles. Filed October 26, 1989 , published April 16, 1991 , Applicant: WR Grace & Co., Inventor: MB Sherwin, J.-L. Su.
- ↑ H. Franzen: About the action of formaldehyde on potassium cyanide . In: J. Prakt. Chem. Band 86 , no. 1 , 1912, p. 133-149 , doi : 10.1002 / prac.19120860108 .
- ↑ Patent US7445917B2 : Process for producing glycolic acid from formaldehyde and hydrogen cyanide. Filed December 21, 2005 , published November 4, 2008 , Applicant: EI du Pont de Nemours and Company, Inventors: R. DiCosimo, A. Panova, JS Thompson, FG Gallagher, T. Foo, X. Li, GC Fox , JJ Zaher, MS Payne, DP O'Keefe.
- ↑ Patent US2890238 : Preparation of glycolonitrile. Applied January 31, 1957 , published June 9, 1959 , Applicant: The Dow Chemical Company, Inventor: AR Sexton.
- ↑ Patent US5208363 : Preparation of aminoacetonitriles. Applied May 5, 1992 , published May 4, 1993 , Applicant: The Dow Chemical Company, Inventor: DK Crump, DA Wilson.
- ↑ Patent US4980471 : Preparation of piperazinones for use as sulfur dioxide absorbents. Filed October 6, 1989 , published December 25, 1990 , Applicant: The Dow Chemical Company, Inventor: SA Christiansen, DA Wilson, D. Chang.
- ↑ Patent EP1004660B1 : An amino nitrile intermediate fort he preparation of 2-hydroxyethyl iminoacetic acid. Filed October 31, 1996 , published November 9, 2005 , applicant: Dow Global Technologies, Inc., inventor: PS Athey, DK Crump, DA Wilson.
- ↑ G. Arrhenius, KK Baldridge, S. Richards-Gross, JS Siegel: Glycolonitrile Oligomerization: Structure of Isolated Oxazolines, Potential Heterocycles on the Early Earth . In: J. Org. Chem. Band 62 , no. 16 , 1997, pp. 5522-5525 , doi : 10.1021 / jo962185r .