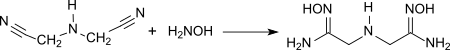Iminodiacetonitrile
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Iminodiacetonitrile | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 5 N 3 | |||||||||||||||
| Brief description |
white crystal powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 95.11 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point | ||||||||||||||||
| solubility | ||||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Iminodiacetonitrile (IDAN) is a water-soluble dinitrile with a secondary amino group , which can also be regarded as a disubstitution product by replacing two hydrogen atoms on the ammonia molecule with –CH 2 CN groups.
IDAN finds u. a. Used as an intermediate product for the chelating ligand diethylenetriamine (DETA) and the total herbicide glyphosate .
Manufacturing
Hexamethylenetetramine as a reactant
The synthesis of iminoacetonitrile from hexamethylenetetramine (as a source for the actual reactants formaldehyde and ammonia ) and hydrogen cyanide was described as early as 1894.
Under the chosen conditions, the reaction requires reaction times of several days and uses only three of the four nitrogen atoms of the hexamethylenetetramine. The Dubsky variant with the addition of hydrochloric acid to bind the ammonia formed, with reaction times of over 24 hours and a yield of approx. 63%, does not represent a significant improvement in the process.
The lack of atom economy in the stoichiometric use of hexamethylenetetramine is addressed by a process variant in which glycolonitrile (HOCH 2 CN) is added to the reaction .
In this process , which is designed as a continuous process, an aqueous solution of the reactants is brought to practically quantitative conversion in a tubular reactor at 120-140 ° C. and a residence time of about 2-3 minutes.
A newer variant of the batch process with hexamethylenetetramine, 50% formalin solution and hydrogen cyanide at a pH of 5.5 and 75 ° C for two hours precipitates out IDAN in a yield of 79% on cooling. After increasing the pH to 7, the filtrate is kept at 75 ° C. for a further two hours, with by-products such as glycolonitrile, aminoacetonitrile and ammonium salts being converted into IDAN. Overall, a total iminodiacetonitrile yield of 97% is achieved in this way.
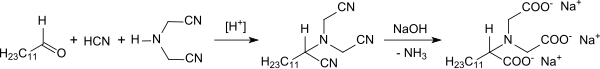
Formaldehyde, hydrogen cyanide and ammonia as reactants
When potassium cyanide was reacted with aqueous formaldehyde solution, in addition to glycolonitrile, the formation of small amounts of iminodiacetonitrile and nitrilotriacetonitrile was found. The ammonia required for the formation of IDAN comes from the hydrolysis of glycolonitrile to glycolic acid . The overall response
however, it does not run uniformly. Due to the complex composition of the products obtained from the reaction of formaldehyde, hydrogen cyanide and ammonia, the influence of the process parameters, ratio of reactants, reaction temperature, pH value, reaction time and batch process versus continuous process was intensively investigated and documented in many process patents.
With a ratio of NH 3 : HCHO: HCN = 2: 3: 3, 0 to 25 ° C reaction temperature and a pH value of 5.5 to 6.5, an IDAN yield of a maximum of 65% with a reaction time of approx Get 20 hours.
Even with a process variant with stoichiometric amounts of the reactants, pH between 3.5 and 5.5, a temperature of 30 to 65 ° C and the replacement of ammonia by ammonium salts, such as. B. ammonium nitrate or ammonium acetate , no more than 74% IDAN yield is achieved.
Instead of aqueous solutions of the (laboriously) purified reactants, the unpurified gas stream from the HCN reactor can also be reacted with the likewise unpurified gas stream from the formaldehyde reactor together with ammonia and acidified water in a reactive gas scrubber at 65 to 70 ° C, IDAN yields of up to 85%, however, with reaction times of over 10 hours can be achieved.
IDAN syntheses from intermediate products of the reaction of HCHO, HCN and NH 3
Aminoacetonitrile and glycolonitrile react within 15 minutes at 140 ° C. to form iminodiacetonitrile in a yield of 78%.
In the absence of glycolonitrile, aminoacetonitrile (AAN) can be converted to an aminonitrile mixture of AAN and IDAN within wide limits of 5 to 70 percent by weight of IDAN by varying the process parameters temperature, reaction time and amount of ammonia.
The conversion of glycolonitrile with ammonia at a pH value of 5.5 to 6 and 70 ° C provides IDAN with a yield of up to 81% in a batch process, while glycolonitrile conversions of over 90% are achieved in the continuous process. In addition to the disubstitution product IDAN, the trisubstitution product nitrilotriacetonitrile (NTAN) and the condensation product methylene-bis [iminodiacetonitrile] (MBIDAN) are formed as by-products in addition to the monosubstitution product aminoacetonitrile.
properties
Iminodiacetonitrile is a crystalline solid that is obtained as a white powder or in long, colorless needles. The compound is harmful and can cause serious skin and eye irritation.
Applications
The hydrolysis of the two nitrile groups of iminodiacetonitrile with z. B. 20% sodium hydroxide solution at 50 ° C and subsequent acidification produces the complexing agent iminodiacetic acid (IDA).
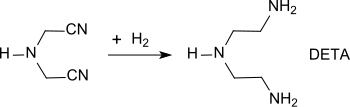
Hydrogenation of a solution of IDAN in tetrahydrofuran in the presence of Raney cobalt at 180 bar and 120 ° C. yields diethylenetriamine (DETA).
The addition of strongly anionic ion exchangers reduces the hydrolysis of IDAN at higher temperatures, whereby DETA selectivities of up to 95% can be achieved.
Long-chain glycine N , N -diacetic acid derivatives based on IDAN have three carboxy groups and are suitable as biodegradable complexing agents for alkaline earth metal and heavy metal ions.
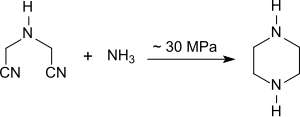
Iminodiacetonitrile can be converted into piperazine with a yield of 85% at approx. 100 ° C. and approx. 300 atm. Pressure within 4 hours in the presence of ammonia and a nickel catalyst .
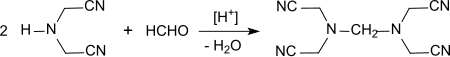
The reaction of IDAN with formaldehyde at a strongly acidic pH (approx. 1) and 50 ° C specifically generates methylene-bis- [iminodiacetonitrile] (MBIDAN), which is a by-product of the IDAN synthesis, in yields of up to 81%.
IDAN reacts with hydroxylamine in 88% yield to form di-amidoxime, which can be used as an effective complexing agent to remove metal contamination in cleaning processes in semiconductor manufacturing.
An alternative synthetic route to the anthelmintic praziquantel
starts from IDAN and leads to the target product over six stages in 35% overall yield.
The quantitatively most important application of iminodiacetonitrile is in the so-called IDAN route - also known as the HCN process - to a key intermediate N -phosphonomethyliminodiacetic acid (PMIDA) of the total herbicide glyphosate (Roundup®).
If inexpensive hydrogen cyanide is available according to the Andrussow process and the use of catalytic air oxidation of PMIDA to glyphosate is the IDAN route compared to the alternative routes DEA process (based on ethylene oxide ) and in particular compared to the older glycine process (based on monochloroacetic acid or Paraformaldehyde ) of economic interest.
Individual evidence
- ↑ a b c d e Entry on iminodiacetonitrile at TCI Europe, accessed on February 10, 2016.
- ↑ a b c data sheet iminodiacetonitrile from AlfaAesar, accessed on August 14, 2020 ( PDF )(JavaScript required) .
- ↑ a b W. Eschweiler: About some acetonitriles . In: Justus Liebigs Ann. Chem. Band 278 , no. 2 , 1894, p. 229-239 , doi : 10.1002 / jlac.18942780207 .
- ↑ a b Patent US2794044 : Synthesis of iminoacetonitrile. Applied on August 30, 1955 , published May 28, 1957 , applicant: EI du Pont de Nemours and Company, inventor: WR Miller.
- ^ JV Dubsky: On the knowledge of the Diketo-piperazine, XI. Communication: E. Dingemanse: 3,5-diketo-1-benzyl- [hexahydro-1,4-diazine] . In: Ber. German chem. Ges. Band 54 , no. 10 , 1921, pp. 2659-2667 , doi : 10.1002 / cber.19210541014 .
- ↑ a b Patent US3904668 : Process for preparing iminodiacetonitrile and alkali metal iminodiacetates. Filed November 15, 1973 , published September 9, 1975 , Applicant: WR Grace & Co., Inventor: RR Gaudette, JE Philbrook, JC Thunberg.
- ↑ a b Patent EP0413673B2 : Process for the preparation of iminodiacetonitrile. Applied on August 13, 1990 , published November 20, 1996 , Applicant: Monsanto Company, Inventor: KE Koenig, PA Morrison, GA Lanser, RB Weisenfeld.
- ↑ K. Polstorff, H. Meyer: About the action of potassium cyanide on formaldehyde . In: Ber. German chem. Ges. Band 45 , no. 2 , 1912, pp. 1905–1912 , doi : 10.1002 / cber.19120450263 .
- ↑ Patent US4661614 : Process for the preparation of iminodiacetonitrile. Applied March 6, 1986 , published April 28, 1987 , Applicant: Monsanto Company, Inventor: JT Most, TJ Richard.
- ↑ Patent US4895971 : Process for the production of iminodiacetonitrile. Filed October 31, 1988 , published January 23, 1990 , applicant: WR Grace & Co.-Conn., Inventor: J.-L. Su, MB Sherwin.
- ↑ a b Patent US2511487 : Synthesis of iminodiacetonitrile. Applied on August 8, 1946 , published June 13, 1950 , applicant: EI du Pont de Nemours & Company, inventor: HT Thompson.
- ↑ Patent US8153845B2 : Method for producing aminonitriles. Registered on February 28, 2008 , published on April 10, 2012 , applicant: BASF SE, inventors: A. Oftring, K. Dahmen, T. Hahn, R. Hugo, K. Baumann, J.-P. Detector.
- ↑ Patent US5187301 : Preparation of iminoacetonitrile from glycolonitrile. Filed October 11, 1990 , published February 16, 1993 , Applicant: WR Grace & Co.-Conn., Inventor: BA Cullen, BA Parker.
- ↑ Patent US7880035B2 : Method for producing ethyleneamines. Registered on February 28, 2008 , published on February 1, 2011 , applicant: BASF SE, inventors: A. Oftring, K. Dahmen, T. Hahn, R. Hugo, K. Baumann, J.-P. Detector.
- ↑ Patent EP2684862A1 : Method for preparing N- (2-aminoethyl) ethane-1,2-diamine. Filed April 1, 2012 , published January 15, 2014 , Applicants: Wanhua Chemical Group Co., Ltd., Ningbo Wanhua Polyurethanes Co., Ltd., Inventors: F. Li, Y. Li, K. Ding, W. Zhao, X. Yu, W. Hua.
- ↑ Patent US6420593B2 : Method for producing long-chain glycine-N, N-diacetic acid derivatives. Registered on June 8, 2001 , published on July 16, 2002 , applicant: BASF AG, inventor: R. Rahm, T. Greindl, A. Oftring, G. Oetter, J. Detering, G. Braun.
- ↑ Patent US2809196 : Synthesis of piperazine. Applied on August 30, 1955 , published October 8, 1957 , applicant: EI du Pont de Nemours & Co., inventor: WR Miller.
- ↑ Patent US3532735 : Preparation of methylenebisiminodiacetonitrile. Applied October 7, 1968 , published October 6, 1970 , applicant: WR Grace & Co., inventor: CR Morgan.
- ↑ Patent US7838483B2 : Process of purification of amidoxime containing cleaning solutions and their use. Filed October 29, 2008 , published November 23, 2010 , applicant: EKC Technology, Inc., inventor: WM Lee, CCY Chen.
- ↑ Patent US4523013 : 4-Acyl-2,6-dioxo-1-phenethyl piperozines. Applied January 31, 1983 , published June 11, 1985 , applicant: Sanofi SA, inventor: D. Fréhel, J.-P. Maffrand.
- ↑ Patent US4724103 : Process for preparing N, N-diacetic acid aminomethylene phosphonic acid. Applied December 5, 1984 , published February 9, 1988 , applicant: Monsanto Co., inventor: MJ Gentilcore.
- ↑ J. Tian, H. Shi, X. Li, Y. Yin, L. Chen: Coupling mass balance analysis and multi-criteria ranking to assess the commercial-scale synthetic alternatives: a case study on glyphosate . In: Green Chemistry . tape 14 , 2012, p. 1990-2000 , doi : 10.1039 / C2GC35349K .