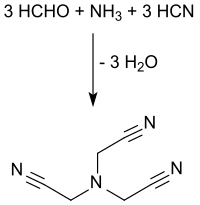
Nitrilotriacetonitrile
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Nitrilotriacetonitrile | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 6 N 4 | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 134.14 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
|
|||||||||||||||
| Melting point |
|
|||||||||||||||
| solubility |
Slightly soluble in water (3 g l −1 at 20 ° C ), soluble in nitromethane and in acetone |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Nitrilotriacetonitrile (NTAN) is a precursor of nitrilotriacetic acid (NTA), a biodegradable chelating agent and builder for detergent , for tris (2-aminoethyl) amine , a tripodal tetradentate, under the abbreviation tren conventional chelating agents , as well as for the epoxy resin - crosslinkers 1- ( 2-aminoethyl) piperazine .
Occurrence and representation
The synthesis of nitrilotriacetonitrile is based on the basic building blocks ammonia , formaldehyde and hydrogen cyanide , which are brought to a reaction - a triple cyanomethylation of ammonia - in an acidic aqueous medium in discontinuous or continuous processes.
Ammonia is provided in gaseous form, as hexamethylenetetramine or in the form of ammonium sulfate together with formaldehyde as an aqueous (usually 37% by weight) formalin solution at pH values <2 and with aqueous hydrocyanic acid solution or liquid hydrogen cyanide - if necessary without pre-cleaning directly from the Andrussow process or the BMA process from Evonik Degussa - implemented at temperatures around 100 ° C. When the mother liquors are recycled, yields of over 90% are achieved.
Particularly problematic when the process is carried out continuously is the tendency of NTAN to precipitate at temperatures below 90.degree. C., which can lead to clogging of tubular reactors and pipes and thermal runaway reaction of the reaction.
properties
Nitrilotriacetonitrile is a colorless and odorless solid that dissolves poorly in water and dissolves well in nitromethane and acetone.
Applications
Nitrilotriacetonitrile can be in the melt in the presence of basic catalysts, such as. B. sodium homo- or iminodiacetonitrile to dark colored solid polymers copolymerize to nitrogenous at temperatures> 1000 ° C under a protective gas and electrically conductive polymers, carbonized blank. The products obtained have found no use as conductive polymers .
The hydrogenation of NTAN first converts a cyano group into an imino group, which attacks an adjacent cyano group sterically suitable for the formation of a six-membered ring system more quickly than being further hydrogenated to the primary amino group . The end product of the catalytic hydrogenation of nitrilotriacetonitrile is therefore 1- (2-aminoethyl) piperazine .
If you carry out the catalytic hydrogenation of NTAN with z. B. Raney nickel, on the other hand, in the presence of a large excess of ammonia, one obtains tris (2-aminoethyl) amine,
a tetradentate complexing agent (abbreviated as "tren"), which forms stable chelates, especially with divalent and trivalent transition metal ions.
Nitrilotriacetonitrile reacts with formaldehyde at pH 9.5 to form 2,2-dihydroxymethyl-nitrilotriacetonitrile, which is hydrolyzed with sodium hydroxide solution at 100 ° C to the trisodium salt of 2-hydroxymethylserine-N, N-diacetic acid, from which the free acid is 51% acidified. iger yield can be obtained.
The compound is suitable as a complexing agent for heavy metal or alkaline earth metal ions, as a bleach stabilizer, e.g. B. for sodium perborate , in solid detergent preparations and as a builder in detergents to inhibit the formation of deposits (incrustations) in textiles during washing.
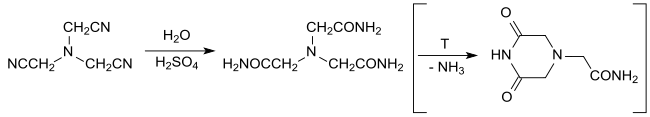
The hydrolysis of nitrilotriacetonitrile with water in concentrated sulfuric acid yields virtually quantitative nitrilotriacetamide under mild conditions, which was investigated as a neutral tetradentate ligand for metal complexation. At elevated temperatures, ring closure produces 3,5- dioxopiperazine- 1-acetamide, which after neutralization and heating with excess aqueous ammonia can be converted quantitatively back into nitrilotriacetamide.
Nitrilotriacetonitrile is mainly used as a raw material for the production of the biodegradable but carcinogenic complexing agent nitrilotriacetic acid through acid or base-catalyzed hydrolysis of the cyano groups.
Undesired residual levels of cyanide ions in the hydrolyzate can be removed by post-treatment with oxidizing agents, such as. B. Sodium hypochlorite at pH 8 can be removed.
Individual evidence
- ↑ a b c d Patent US3337607 : Process for preparation of an amine nitrile. Filed January 25, 1965 , published August 22, 1967 , Applicant: Ethyl Corp., Inventor: JC Wollensak.
- ^ Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Oxford, UK 2014, ISBN 978-0-323-28659-6 , pp. 279 .
- ↑ a b c Patent US3840581 : Process for the manufacture of nitrilotriacetonitrile. Registered on May 26, 1970 , published October 8, 1974 , applicant: Knapsack AG, inventors: H. Neumaier, W. Vogt, K. Sennewald, R. Schuller, G. Lenz.
- ↑ a b M.D. Larranaga, RJ Lewis, Sr., RA Lewis: Hawley's Condensed Chemical Dictionary, 16th Edition . Wiley, Hoboken, NJ 2016, ISBN 978-1-118-13515-0 , pp. 974 .
- ↑ Registration dossier on nitrilotriacetonitriles (section Stability in organic solvents and identity of relevant degradation products ) at the European Chemicals Agency (ECHA), accessed on December 26, 2017.
- ↑ a b Pfaltz & Bauer: N05890, Nitrilotriacetonitrile , accessed on December 26, 2017.
- ↑ Patent US3061628 : Process and preparation of amino nitriles and acetic acids. Filed September 12, 1958 , published October 30, 1962 , Applicant: Hampshire Chemical Corp., Inventor: JJ Singer, Jr., M. Weisberg.
- ↑ Patent EP0102343B1 : Process for producing nitrilotriacetonitrile. Applied on August 25, 1983 , published on February 26, 1986 , applicant: Monsanto Co., inventor: CY Shen.
- ^ E. Fiedler: Emergency Runaway Reaction - What Precedes? What Follows? In: Chem. Engineer. Transactions (CET) . tape 48 , 2016, ISBN 978-88-95608-39-6 , pp. 361-366 , doi : 10.3303 / CET1648061 .
- ↑ Product Stewardship Summary, chelate: NTAN. (PDF; 45.7 KB) (No longer available online.) In: akzonobel.com. Akzo Nobel Functional Chemicals, archived from the original on June 2, 2013 ; accessed on March 20, 2017 (English). Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Patent US3578643 : New polymers from nitrilotriacetonitrile and iminodiacetonitrile. Filed June 6, 1969 , published May 11, 1971 , applicant: WR Grace & Co., inventor: LL Wood, RA Hamilton.
- ↑ Patent US3565957 : Hydrogenation of nitrilotriacetonitrile. Applied September 20, 1968 , published February 23, 1971 , Applicant: Stauffer Chemical Co., Inventor: SB Mirviss, DJ Martin, ED Weil.
- ^ G. Anderegg, V. Gramlich: 1: 1 Metal Complexes of Bivalent Cobalt, Nickel, Copper, Zinc, and Cadmium with the Tripodal Ligand tris [2- (dimethylamino) ethyl] amine: Their stabilities and the X-ray crystal structure of its copper (II) complex sulfate . In: Helv. Chim. Acta . tape 77 , no. 3 , 1994, p. 685-690 , doi : 10.1022 / hlca.19940770312 .
- ↑ Patent EP0396999A2 : 2-methyl- and 2-hydroxymethyl-serine-N, N-diacetic acid and its derivatives. Applied on April 30, 1990 , published on November 14, 1990 , applicant: BASF AG, inventor: A. Oftring, S. Birnbach, R. Bauer, C. Gousetis, W. Trieselt.
- ↑ DA Smith, S. Sucheck, S. Cramer, D. Baker: Nitrilotriacetamide: Synthesis in Concentrated sulfuric acid and stability in water . In: Synth. Commun. tape 25 , no. 24 , 1995, pp. 4123-4132 , doi : 10.1080 / 00397919508011491 .
- ↑ Patent GB1170399 : A process for preparing 3,5-dioxo-1-piperazineacetamide and nitrilotriacetic acid triamide. Filed on 12 June 1968 , published on November 12, 1969 , Applicant: WR Grace & Co ..
- ↑ DA Smith, S. Cramer, S. Sucheck, E. Skrzypzak-Jankun: Facile synthesis of substituted nitrilotriacetamides . In: Tetrahedron Lett. tape 33 , no. 50 , 1992, pp. 7765-7768 , doi : 10.1016 / 0040-4039 (93) 88040-P .
- ↑ a b Patent US8362298B2 : Hydrolyzed nitrilotriacetonitrile compositions, nitrilotriacetonitrile hydrolysis formulations and methods for making and using the same. Filed May 20, 2011 , published January 29, 2013 , applicant: Clearwater International, LLC, inventor: OM Falana, A. Hikem, SR Kakadjian, F. Zamora.
- ↑ Patent US4547589 : Hydrolysis of nitrilotriacetonitrile. Applied January 3, 1984 , published October 15, 1985 , applicant: Monsanto Co., inventor: CY Shen.