Trioxane
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
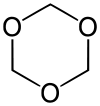
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Trioxane | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 3 H 6 O 3 | ||||||||||||||||||
| Brief description |
Highly flammable, white solid with an odor similar to ethanol |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 90.08 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.17 g cm −3 (65 ° C) |
||||||||||||||||||
| Melting point |
62 ° C |
||||||||||||||||||
| boiling point |
115 ° C |
||||||||||||||||||
| Vapor pressure |
11 h Pa (20 ° C) |
||||||||||||||||||
| solubility |
good in water (221 g l −1 at 25 ° C) |
||||||||||||||||||
| Dipole moment | |||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−522.5 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
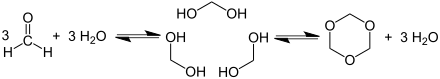
Trioxane is a heterocyclic compound from the acetals group , which is formed by the trimerization of formaldehyde . The IUPAC name is 1,3,5-trioxacyclohexane or 1,3,5-trioxane for short . The cyclic trimers of other aldehydes are also known as trioxanes .
Presentation, properties and use
Trioxane is produced using acid catalysis by trimerizing formaldehyde.
It is also produced directly from methanol by dehydrogenation or oxidation with atmospheric oxygen. The highly flammable substance forms colorless needles that dissolve well in water, alcohol and ether. When heated to 150 to 200 ° C in the presence of water, trioxane depolymerizes again to form monomeric formaldehyde. This is a potential hazard, especially if dry fuels containing trioxane are improperly stored, see Esbit cooker . Because of the catalytic effect of basic substances, cleaning agents in particular must be stored separately from dry fuels.
Trioxane is used in the manufacture of polyacetal plastics. Trioxane is also used in syntheses that start from formaldehyde and require it in a very pure form.
Safety instructions / risk assessment
Trioxane is very flammable. When mixed with air, the vapors are explosive in the wide concentration range from 3.6 to 29% by volume. Trioxane irritates skin, mucous membranes and eyes and can damage the respiratory tract and the central nervous system if the vapors are inhaled .
In 2012, trioxane was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of trioxane were concerns regarding environmental exposure , high (aggregated) tonnage and widespread use, as well as the dangers arising from a possible assignment to the group of CMR or PBT / vPvB substances and the possible risk from sensitizing properties. The re-evaluation took place from 2013 and was carried out by Poland . A final report was then published in which it was recommended that trioxane should also be classified in category 2 - eye irritant .
Individual evidence
- ↑ a b c d e f g h i j Entry on 1,3,5-trioxane in the GESTIS substance database of the IFA , accessed on May 2, 2020(JavaScript required) .
- ↑ a b Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): 1,3,5-trioxane , accessed on March 26, 2019.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dipole Moments, pp. 9-58.
- ↑ Entry on 1,3,5-trioxane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-24.
- ↑ a b Entry on formaldehyde. In: Römpp Online . Georg Thieme Verlag, accessed on February 28, 2014.
- ^ European Chemicals Agency (ECHA): Substance Evaluation Report and Conclusion Document .