Pentaerythritol trinitrate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
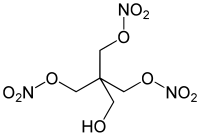
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Pentaerythritol trinitrate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 9 N 3 O 10 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 271.14 g mol −1 | |||||||||||||||
| Melting point |
<25 ° C |
|||||||||||||||
| solubility |
slightly soluble in water: 7050 mg l −1 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Pentaerythritol trinitrate (Petrin) is a little-known yet powerful explosive with a detonation speed of up to 8450 m · s −1 . Like the more common pentaerythritol granitrate known as nitropenta , trinitrate also has a strong vasodilatory effect .
Manufacture and composition
Pentaerythritol trinitrate is a nitrate (an ester of nitric acid) of pentaerythritol. It occurs either as a by-product in the classic esterification of pentaerythritol with nitric acid or is obtained by nitration of pentaerythritol with a mixed acid. A small part of the tetranitrate is produced there.
properties
The detonation speed is at a maximum density of 1.54 g / cm³ up to 8450 m · s −1 . In addition, pentaerythritol trinitrate has a molar mass of 271.14 g / mol and an oxygen balance of −26.6%.
The biggest disadvantage is the shorter storage time of 5 to 10 years compared to Nitropenta.
Legal position
The handling, traffic, transport and import of Petrin are subject to the Explosives Act .
Individual evidence
- ↑ a b Entry on pentaerythritol trinitrate in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on November 27, 2018.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Josef Köhler, Rudolf Meyer, Axel Homburg: Explosivstoffe. 10th edition, Wiley-VCH, Weinheim 2008, ISBN 978-3-527-32009-7 , p. 231.