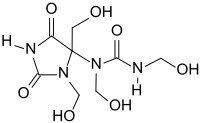
Diazolidinyl urea
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Diazolidinyl urea | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 8 H 14 N 4 O 7 | |||||||||||||||||||||
| Brief description |
white crystal powder |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 278.22 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point | ||||||||||||||||||||||
| solubility |
very easily soluble in water |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Diazolidinyl urea is the essential component in the condensation product of allantoin with formaldehyde and a so-called formaldehyde releaser . The compound is used as a biocide to preserve cosmetics and personal care products.
Occurrence and representation
Allantoin suspended in excess formaldehyde as a 37% solution (formalin) reacts in an alkaline environment at 100 ° C to form various soluble condensation products that arise as a white solid during acidification and concentration.
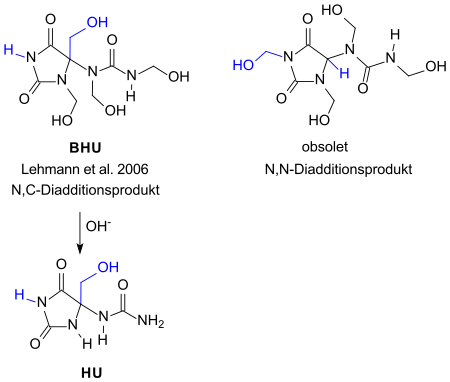
The reaction mixture contains about 30 to 40% of the starting material allantoin and the condensation products HU and in particular the target product BHU . The target product differs in the substitution pattern on the imidazolidine ring from the previously stated structure of a 1- [1,3-bis (hydroxymethyl) -2,5-dioxoimidazolidin-4-yl] -1,3-bis (hydroxymethyl) urea. The now obsolete structural formula can still be found in many publications. In addition, non-characterized allantoin-formaldehyde polymers are produced .
Exact adherence to a molar ratio of allantoin to formaldehyde = 1: 4 should yield a uniform condensation product, the structural formula of which is also given in the patent as N , N - instead of N , C - bis (hydroxymethyl) imidazolidine diaddition product.
properties
Diazolidinyl urea is a white, very water-soluble powder with a faint characteristic odor. The stated melting points, which differ greatly from one another, indicate an inconsistent composition of the product. Only about 50% of the theoretical amount of formaldehyde can be released from diazolidinyl urea. The substance is stable over a wide pH range from 3 to 9 and is compatible with many cosmetic ingredients and preservatives. Diazolidinyl urea is degraded to 4- (hydroxymethyl-2,5-dioxo-imidazolidinyl) urea HU under basic and neutral conditions . It has a bactericidal effect against gram-positive and gram-negative bacteria, but less so against molds and yeasts.
Applications
Diazolidinylurea was patented in 1966 and introduced in 1982 as a formaldehyde releaser or formaldehyde donor because of its biocidal effect for the preservation of personal care products and cosmetic preparations. In order to avoid decomposition and the associated release of formaldehyde, products containing diazolidinyl urea should not be processed and stored above 40 ° C. Typical concentrations in preparations are between 0.1 and 0.3%, with maximum concentrations of 0.5% permitted in the EU and the USA. In Japan, diazolidinyl urea is not permitted as a preservative. To increase the fungicidal effect, mixtures with parabens or iodocarb are often used.
Manufacturer and trade name
Diazolidinyl urea is manufactured and marketed by Ashland, Inc. under the brand name Germall II®, by 3V Sigma under the brand name Abiol ™ Forte, and by Vantage Specialty Ingredients under the brand name Liposerve ™ DU.
safety instructions
In contrast to many other formaldehyde releasers, diazolidinyl urea is practically only added to cosmetic products for preservation. The acute and subchronic oral and dermal toxicity values of diazolidinyl urea are toxicologically insignificant and Ames tests for mutagenicity yielded negative results . However, more recent findings from in vitro experiments indicated genotoxicity . The Cosmetic Ingredient Review (CIR) Expert Panel decided in 2006 not to restart the evaluation of diazolidinyl urea.
The available data on sensitization to diazolidinyl urea, which have a prevalence of 0.8 to 1.8%, appear to be more problematic ; according to another source, 0.5 to 1.4% in Europe. In the case of long-acting formaldehyde releasers with inconsistent composition, e.g. B. also 3,3'-methylenebis (5-methyloxazolidine) , a monocausal connection with the active ingredient, one of the by-products, the released formaldehyde or one of the degradation products is hardly conclusive. Because of the mostly repetitive and long-term external use of (biocide-containing) cosmetics, the occurrence of allergies when using formaldehyde releasers requires special attention.
literature
- Heather AE Benson, Michael S. Roberts, Vania R. Leite-Silva, Kenneth A. Walters (Eds.): Cosmetic Formulation: Principle and Practice . CRC Press, Boca Raton, FL, USA 2019, ISBN 978-1-4822-3539-5 , pp. 196-197 .
Individual evidence
- ↑ Entry on DIAZOLIDINYL UREA in the CosIng database of the EU Commission, accessed on March 30, 2020.
- ↑ a b c d e f Entry on 1- (1,3-bis (hydroxymethyl) -2,5-dioxoimidazolidin-4-yl) -1,3-bis (hydroxymethyl) urea in the GESTIS substance database of the IFA , retrieved on March 30, 2020 (JavaScript required)
- ↑ Diazolidinyl urea data sheet (PDF) from Fisher Scientific , accessed March 30, 2020.
- ↑ a b data sheet diazolidinyl urea from Sigma-Aldrich , accessed on April 02, 2020 ( PDF ).
- ↑ Patent US3248285 : Allantoin-formaldehyde condensation products. Filed August 16, 1962 , published April 26, 1966 , Assignee: Sutton Laboratories, Inc., Inventor: PA Berke.
- ^ SV Lehmann, U. Hoeck, J. Breinholdt, CE Olsen: FS05.3 New characterization and chemistry of Germall 115 and Germall II . In: Contact Dermatitis . tape 50 , no. 3 , 2004, p. 144–145 , doi : 10.1111 / j.0105-1873.2004.0309bf.x .
- ^ SV Lehmann, U. Hoeck, J. Breinholdt, CE Olsen: Characterization and chemistry of imidazolidinyl urea and diazolidinyl urea . In: Contact Dermatitis . tape 54 , no. 1 , 2006, p. 50-58 , doi : 10.1111 / j.0105-1873.2006.00735.x .
- ↑ a b c d A.C. de Groot, IR White, MA Flyvholm, G. Lensen, PJ Coenraads: Formaldehyde-releasers in cosmetics: relationship to formaldehyde contact allergy. Part 1. Characterization, frequency and relevance of sensitization, and frequency of use in cosmetics . In: Contact Dermatitis . tape 62 , no. 1 , 2010, p. 2–17 , doi : 10.1111 / j.1600-0536.2009.01615.x .
- ↑ Patent WO198100566 : N- (hydroxymethyl) -N- (1,3-dihydroxymethyl-2,5-dioxo-4-imidazolidinyl) -N '- (hydroxymethyl) urea, preservative compositions and use thereof. Filed August 28, 1979 , published March 5, 1981 , Applicant: Sutton Laboratories, Inc., Inventor: PA Berke, WE Rosen.
- ↑ a b M.A. Liebert: Final Report on the Safety Assessment of Diazolidinyl Urea . In: J. Am. Coll. Toxicol. tape 9 , no. 2 , 1990, p. 229-245 , doi : 10.3109 / 10915819009078735 .
- ^ AC de Groot, IR White, M.-A. Flyvholm, G. Lensen, P.-J. Coenraads: Formaldehyde-releasers: relationship to formaldehyde contact allergy. Contact allergy to formaldehyde and inventory of formaldehyde-releasers . In: Contact Dermatitis . tape 61 , no. 2 , 2009, p. 63-85 , doi : 10.1111 / j.1600-0536.2009.01582.x .
- ↑ S. Pfuhler, HU Wolf: Effects of the formaldehyde-releasing preservatives dimethylol urea and Diazolidinyl Urea in several short-term genotoxicity tests . In: Mutat. Res. Band 514 , no. 1–2 , 2002, pp. 133-146 , doi : 10.1016 / s1383-5718 (01) 00335-7 .
- ↑ Annual Review of Cosmetic Ingredient Safety Assessments: 2005/2006 . In: Int. J. Toxicol. tape 27 , 2008, p. 77-142 , doi : 10.1080 / 10915810802032362 .
- ↑ Formaldehyde and Formaldehyde Releasers. ECHA European Chemicals Agency, March 15, 2017, accessed March 31, 2020 .
- ↑ James G. Marks, Jr., Bryan E. Anderson, Vincent A. DeLeo: Contact amd Occupational Dermatology, 4th ed. Jaypee Brothers Medical Publishers, New Delhi 2016, ISBN 978-93-5152936-1 , pp. 82-83 .