Schiff's sample
The Schiff's sample is a chemical reaction named after its German-Italian discoverer, Hugo Schiff , which is used to detect aldehydes .
Working principle
Aldehydes react with the amino groups of the initially colorless Fuchsinschwefligen acid, an adduct of sulfuric acid at Fuchsin , including restoration of the original sp (s u..) 2 - hybridization of its central carbon atom to again colored pink to violet triphenylmethane ( bathochromic shift ). The exact mechanism of the reaction, which took place over several intermediate stages, could not be elucidated until 1980 by means of NMR spectroscopy . Other compounds are regularly formed as by-products, for example through competitive reaction of the aldehyde used with sulphurous acid with the formation of hydroxyalkyl sulphonate ions (cf. bisulphite reaction ). In the reaction of Schiff's reagent e.g. B. with formaldehyde - depending on the reaction mixture and conditions - at least 3 to 6 different compounds can be formed.
Manufacture of Schiff's reagent
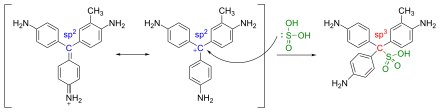
The reagent is prepared by adding sulfurous acid (aqueous sulfur dioxide solution) drop by drop to an aqueous fuchsin solution until the red color of the fuchsin has disappeared. Alternatively, sulphurous acid can be produced in situ from sodium hydrogen sulphite solution (NaHSO 3 ) (aq) by acidification. The discoloration of the fuchsin is caused by the fact that the sulfur atom of the sulphurous acid binds to the central carbon atom of the fuchsin and thus interrupts its mesomeric electron system, with the result of the observed loss of color ( hypsochromic effect ).
selectivity
The reaction does not proceed with sugars and glyoxal , aromatic hydroxy aldehydes and α, β-unsaturated aldehydes. In contrast to Fehling's solution and Tollens reagent , the reducing effect of the aldehydes is not used for their detection.
Areas of application
In histology and pathology , PAS staining is used, which can stain glycogen , cellulose , neutral proteoglycans (glycosaminoglycans / mucopolysaccharides), mucoproteins and glycoproteins with periodic acid and Schiff's sample .
Since human skin contains aldehydes, colored spots will also appear on skin contact with the reagent.
In analytics, fuchsin sulphurous acid is used for the qualitative detection of volatile oxidizing agents such as bromide, which is oxidized to bromine and detected on a filter paper soaked with Schiff's reagent. The sulphite group is oxidized to sulphate and split off: Colored fuchsin is formed from the colorless fuchsin-sulphurous acid.
In school chemistry the negative course of the Schiff's test with glucose is often interpreted as an indication that the aldehyde group of this molecule is bound in the form of an intramolecular hemiacetal and therefore cannot react with the fuchsin sulphurous acid. More recent findings on the reaction mechanism of the Schiff's sample, on the other hand, suggest that the aldehyde group of glucose is blocked by competing hydrogen sulfite.
Individual evidence
- ↑ Wieland, H. and Scheuing, G .: The fuchsin sulfurous acid and its color reaction with aldehydes. In: Ber. dtsch. Chem. Ges. A / B . tape 54 , no. 10 , 1921, pp. 2527 , doi : 10.1002 / cber.19210541002 .
- ↑ JH Robins, GD Abrams, JA Pincock: The structure of Schiff reagent aldehyde adducts and the mechanism of the Schiff reaction as determined by nuclear magnetic resonance spectroscopy . In: Canadian Journal of Chemistry . tape 4 , no. 58 , 1980, pp. 339–347 , doi : 10.1139 / v80-055 ( PDF ). , last accessed April 20, 2015.
- ↑ Barka T. and Ornstein, L .: SOME OBSERVATIONS OF THE REACTION OF SCHIFF REAGENT WITH ALDEHYDES . In: J Histochem Cytochem . tape 8 , no. 3 , 1960, p. 212 , doi : 10.1177 / 8.3.208 .
- ↑ European Pharmacopoeia , 7th edition.
- ↑ Peter Imming: Pharmacopoeia Analysis - Basics for Study and Practice . 1st edition. Wissenschaftliche Verlagsgesellschaft mbH Stuttgart, 2006, ISBN 978-3-8047-2245-3 , p. 76 f .
- ↑ Karlheinz Seifert: Why does glucose (as an aldose) not react or only weakly with Schiff's reagent? ; University of Bayreuth, 2005 , last accessed April 19, 2015.
Web links
- Peter Keusch: Detection of aldehydes with Schiff's reagent ( Memento from November 14, 2012 in the Internet Archive )

