Carbinols
| Carbinols |
|---|
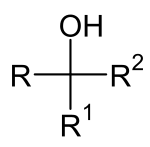
|
| General structure of a carbinol, where R, R 1 and R 2 can be H , aliphatics , aromatics or other radicals. |
Carbinols (also carbinols ) is an outdated term for mostly secondary or tertiary , less often primary alcohols , which are understood as derivatives of methanol (old name carbinol ). The name is made up of the substituents on the central carbon atom and the group name -Carbinol . The name carbinol was proposed by Hermann Kolbe in 1864 . Kolbe referred to the non-existing methyl group CH 3 - as carbine. In order not to have to use the “inconveniently long word carbinoxyhydrate” , he introduced the term carbinol. This designation no longer corresponds to the IUPAC nomenclature rules since 1957 .
Examples
| Surname | Carbinol | structure | Meaning / use |
|---|---|---|---|
| Fenipentol | Phenyl-butyl-carbinol |
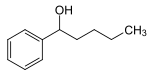
|
Drug ( choleretic ) |
| p-tolylmethylcarbinol | 4-tolyl-methyl-carbinol |

|
|
| Fenarimol | 2-chlorophenyl-5-pyrimidyl-4-chlorophenyl-carbinol |
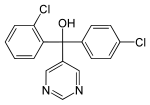
|
fungicide |
| Furfuryl alcohol | α-Furfuryl-carbinol 1-Furfuryl-carbinol |

|
Solvent , wood modification |
| Indole-3-carbinol | 3-indyl-carbinol |

|
Possible drug (anti- carcinogen ) |