Serinweg
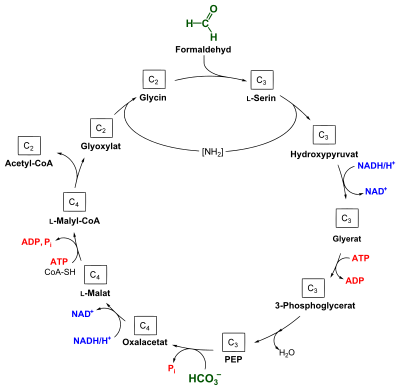
The serine pathway is used by methanotrophic bacteria to synthesize cell material from formaldehyde and bicarbonate . Acetyl-CoA is formed from these two C 1 compounds . The metabolic pathway was identified in type II methanotrophs, whereas type I methanotrophs assimilate formaldehyde via the ribulose monophosphate pathway .
The pathway was identified through the work of John Rodney Quayle and co-workers in Methylobacterium extorquens AM1 in the early 1960s.
biochemistry
In one cycle, formaldehyde (CH 2 O) and bicarbonate (HCO 3 - ) are fixed to acetyl-CoA . CH 2 O often comes from the aerobic oxidation of methane , but it can also be used directly.
In the first step of assimilation, glycine is reacted with formaldehyde to form L - serine , with formaldehyde bound to N 5 , N 10 -methylene tetrahydrofolate . This step is catalyzed by a serine hydroxymethyl transferase (also serine transhydroxymethylase; EC 2.1.2.1 ). In the following process, L -serine is deaminated by a transaminase ( EC 2.6.1.45 ) and converted to phosphoenolpyruvate (PEP) in further steps . This requires energy in the form of one molecule of ATP and two reduction equivalents in the form of NADH . A PEP carboxylase ( EC 4.1.1.31 ) transfers a molecule of HCO 3 - to PEP, whereby oxaloacetate is formed. This is converted into L- malyl-CoA by means of coenzyme A through the consumption of NADH and ATP .
Malyl-CoA is finally cleaved to acetyl-CoA and glyoxylate by a malyl-CoA lyase ( EC 4.1.3.24 ). In order to close the cycle, glyoxylate is aminated to glycine, the amino group required for this comes from the deamination reaction from serine to hydroxypyruvate (see also figure).
Acetyl-CoA is then further assimilated either via the glyoxylate cycle or via the ethylmalonyl-CoA pathway .
Balance sheet
The consumption of ATP and reduction equivalents is lower compared to other CO 2 fixation routes. This is because formaldehyde is more reduced than CO 2 or HCO 3 - . The overall reaction is based on:
literature
- Georg Fuchs (ed.), Hans. G. Schlegel (Author): General Microbiology . Thieme Verlag Stuttgart, 8th edition 2007, ISBN 3-13-444608-1 , pp. 250-251.
- Michael T. Madigan and John M. Martinko: Brock Microbiology . Pearson Studies; 11th updated edition 2009; ISBN 978-3-8273-7358-8 , pp. 657-658.
Individual evidence
- ↑ Large, PJ., Peel, D. and Quayle, JR. (1961): Microbial growth on C1 compounds. II. Synthesis of cell constituents by methanol- and format-grown Pseudomonas AM 1, and methanol-grown Hyphomicrobium vulgare . In: Biochem J . 81; Pp. 470-480; PMID 14462405 ; PMC 1243367 (free full text).
- ↑ Large, PJ., Peel, D. and Quayle, JR. (1962): Microbial growth on C (1) compounds. 3. Distribution of radioactivity in metabolites of methanol-grown Pseudomonas AM1 after incubation with [C] methanol and [C] bicarbonate . In: Biochem J . 82 (3); Pp. 483-488; PMID 16748943 ; PMC 1243485 (free full text).
- ↑ Large, PJ., Peel, D. and Quayle, JR. (1962): Microbial growth on C (1) compounds. 4. Carboxylation of phosphoenolpyruvate in methanol-grown Pseudomonas AM1 . In: Biochem J . 85 (1); Pp. 243-250; PMID 16748968 ; PMC 1243936 (free full text).
- ^ Large, PJ. and Quayle, JR. (1963): Microbial growth on C (1) compounds. 5. Enzyme activities in extracts of Pseudomonas AM1. In: Biochem J. 87 (2); Pp. 386-396; PMID 16749008 ; PMC 1201906 (free full text).
See also
Web links
- Serine pathway in Coxiella burnetii RSA 493 (PATRIC)