Ammonium dihydrogen phosphate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 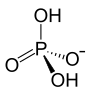
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ammonium dihydrogen phosphate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | (NH 4 ) H 2 PO 4 | |||||||||||||||
| Brief description |
colorless and odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 115.03 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.81 g cm −3 |
|||||||||||||||
| Melting point |
190 ° C (decomposition) |
|||||||||||||||
| solubility |
easily in water (368 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ammonium dihydrogen phosphate (abbreviated MAP from monoammonium phosphate ) is an ammonium salt of phosphoric acid , consisting of the ions NH 4 + (ammonium ion) and H 2 PO 4 - ( dihydrogen phosphate ion ). It forms colorless tetragonal crystals that dissolve well in water.
synthesis
Ammonium can be explained by the neutralization of ammonia with phosphoric win.
use
Ammonium dihydrogen phosphate is mainly used as a fertilizer . It is also suitable for growing crystals . It is also used as an ingredient in fire extinguishing powders.
Individual evidence
- ↑ a b c d e Entry on ammonium dihydrogen phosphate in the GESTIS substance database of the IFA , accessed on July 31, 2019 (JavaScript required)
- ↑ IPNI Nutrient Source Specifics : Monoammonium Phosphate (MAP)

