Hydromorphone
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
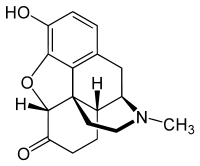
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Hydromorphone | ||||||||||||
| other names | |||||||||||||
| Molecular formula |
|
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| properties | |||||||||||||
| Molar mass | |||||||||||||
| Physical state |
Solid |
||||||||||||
| Melting point | |||||||||||||
| pK s value |
8.2 (20 ° C) |
||||||||||||
| solubility |
|
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Hydromorphone is a powerful semi - synthetic opioid of level III in the WHO level scheme (classification of pain therapy), which is approved as a pain reliever for severe and severe pain . It requires a prescription and is subject to the Narcotics Act .
Pharmacological data
Hydromorphone is a drug that is structurally related to morphine . Chemically, it is a hydrogenated morphine ketone , physiologically a metabolite of morphine, codeine and dihydrocodeine . After resorption, hydromorphone is metabolized in the liver and is then about 50 percent bioavailable. There is a protein binding of approx. Seven percent, the maximum plasma concentration is reached after approx. One hour, the terminal β half-life is approx. Two and a half hours, the excretion is mainly renal . Whether hydromorphone is broken down via the cytochrome P450 system has not yet been clearly established. The water-soluble hydrochloride of hydromorphone is used medicinally; In the therapy, injection solutions (with 2 or 10 mg active ingredient per milliliter) as well as rapid-release (1.3 or 2.6 mg active ingredient) and retarded (2-64 mg containing) capsules and tablets are used. The pain-relieving effect of the injection solution lasts for about one to six hours, that of non-release hard capsules for four and that of sustained-release capsules for 8-12 hours.
Mechanism of action
Opioid analgesics mimic the effects of endogenous opioid peptides ( endorphins , enkephalins , dynorphins ). This on the one hand inhibits the transmission of the pain stimulus and on the other hand changes the pain sensation in the thalamus and in the limbic system .
The pharmacological effect of hydromorphone is similar to that of morphine. It is about 7.5 times as potent (with an initial single dose of 0.2 to 4 mg) compared to morphine .
The metabolism takes place mainly via glucuronidation , the main metabolite is hydromorphone-3-glucuronide - unlike morphine, 6-glucuronide cannot be formed. Since hydromorphone-3-glucuronide is ineffective, hydromorphone can also be given if the kidney function is severely impaired. Because of its low plasma protein binding, the active ingredient has only minor interactions with other drugs. In particular, the sedative effect is significantly less than that of morphine.
Interactions
Hydromorphone increases the effects of substances that dampen the CNS . These include in particular tranquilizers , anesthetics , sleeping pills , sedatives , alcohol , muscle relaxants , antidepressants and antihypertensive drugs .
Side effects
As with all potent opioid analgesics, constipation , nausea, and vomiting can occur. It can also lead to drowsiness , mood changes and changes in the hormonal system and the autonomic nervous system . Overdosing can lead to miosis , hypoventilation and low blood pressure .
Due to its euphoric effect, hydromorphone, as a pure agonist, has a high potential for addiction, especially if it is not used as intended.
Others
Hydromorphone was administered in combination with midazolam in the US state of Ohio in 2014 as an execution poison instead of the otherwise used pentobarbital .
The introduction of a 14 β -ethyl group into the hydromorphone molecule increases the analgesic potency extremely sharply. 14 β- ethyl hydromorphone is 7,500 times more potent than morphine in mice. The unhydrogenated analogue (14 β -ethylmorphinone) even has 10,000 times the potency of morphine.
Commercial preparations
Hydal (A), Jurnista (D, A, CH), Palladon (D, CH) and Generika, Dilaudid (USA, CDN) Hydromorphone Aristo long (D) Hydromorphone (A)
Web links
- Hydromorphone . In: Erowid . (English)
Individual evidence
- ^ A b The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals , 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; P. 832, ISBN 978-0-911910-00-1 .
- ↑ Sean Sweetman (Editor): Martindale: The Complete Drug Reference, 35th Edition: Book and CD-ROM Package . Pharmaceutical Press, ISBN 978-0-85369-704-6 .
- ↑ a b Entry on hydromorphone. In: Römpp Online . Georg Thieme Verlag, accessed on June 12, 2014.
- ↑ a b Datasheet Hydromorphone hydrochloride from Sigma-Aldrich , accessed on April 4, 2011 ( PDF ).
- ↑ Entry on hydromorphone in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Eberhard Klaschik : Pain therapy and symptom control in palliative medicine. In: Stein Husebø , Eberhard Klaschik (ed.): Palliative medicine. 5th edition, Springer, Heidelberg 2009, ISBN 3-642-01548-4 , pp. 207-313, here: p. 233.
- ↑ Eberhard Klaschik : Pain therapy and symptom control in palliative medicine. 2009, p. 233.
- ↑ G. Lindena, H. Arnau, J. Liefhold: Hydromorphone - pharmacological properties and therapeutic effectiveness. In: The Pain, July 1998, Volume 12, Issue 3, pp. 195-204, doi : 10.1007 / s004820050142 .
- ↑ Bischoff, Angelika: In the case of morphine side effects: It makes sense to switch to an extended-release preparation. In: Dtsch Arztebl 1998; 95 (26): A-1686 / B-1427 / C-1333 (June 26, 1998).
- ↑ Pain therapy with opioids , Der Arzneimittelbrief, No. 45/2011, accessed on February 7, 2014.
- ^ Robert M. Julien, et al .: A Primer Of Drug Action , Worth Publishers, 2008, ISBN 978-1-4292-3343-9 .
- ↑ Spiegel Online: Execution in Ohio: New lethal injection caused death row inmates to suffer ten minutes , January 16, 2014, accessed January 17, 2014.
- ^ W. Fleischhacker, B. Richter: Sci. Pharm. 1981, 49, 118; WR Buckett: Brit. J. Pharmacol. 1982, 76, 269P.