Ketobemidone
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
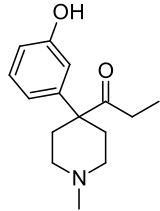
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Ketobemidone | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 15 H 21 NO 2 | |||||||||||||||||||||
| Brief description |
White, crystalline powder (ketobemidone hydrochloride) |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 247.34 g · mol -1 | |||||||||||||||||||||
| Melting point | ||||||||||||||||||||||
| solubility |
soluble in water , soluble in ethanol (hydrochloride) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Ketobemidone (also cetobemidone ) is a fully synthetic opioid from the group of pethidines with a strong analgesic effect. Ketobemidone is a morphine - like piperidine derivative and a pure agonist at the μ-opioid receptor .
description
Ketobemidone was patented in 1947 and 1948 as a strong analgesic by Ciba ( Cliradon ) and also by Winthrop and IG Farben . In the Federal Republic of Germany, ketobemidone is a marketable but not a prescription narcotic due to its listing in Annex II to Section 1 (1) of the BtMG .
chemistry
structure
Dissolved ketobemidone exists in two conformers with an equatorial or axial position of the phenyl ring on the piperidine ring . The latter is considered to be the active conformation that acts on the receptor.
Extraction and presentation
Ketobemidone is produced fully synthetically.
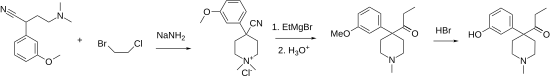
Another synthetic route starts from 3-methoxybenzyl cyanide, which condenses with N , N- bis (2-chloroethyl) - N -methylamine in the presence of the strong base sodium amide to give the methylpiperidine derivative, which reacts with the Grignard reagent methylmagnesium bromide to form an intermediate, which after Cleavage with hydrobromic acid leads to ketobemidone.
pharmacology
effect
As an opioid, ketobemidone has the same profile of effects and side effects, and thus essentially the same risk potential as other opioids. Ketobemidone has an analgesic potency of 1 to 2 and works for four to five hours with a single dose of 5 to 15 mg.
Side effects
These are tiredness, insomnia , drowsiness , nausea , vomiting , edema in the legs, urinary retention , constipation and pruritus . They usually go away as tolerance develops or the dose is reduced. Sleep and sexual disorders last the longest .
Use during pregnancy and breastfeeding: Ketobemidone has an effect on the fetus when taken during pregnancy.
Trade names
Monopreparations : Cliradon (except for trade), Ketodur, Ketogan Novum, Ketorax
literature
- Hermann PT Ammon et al .: Hunnius Pharmaceutical Dictionary . 9th edition, Walter de Gruyter Verlag , Berlin - New York, 2004, ISBN 3-11-017475-8 geb. and ISBN 3-11-017487-1 brosch., p. 327.
- Römpp Lexikon Chemie , Georg Thieme Verlag , Stuttgart - New York, 1997, p. 649 f.
- The Merck Index, 15th edition, The Royal Society of Chemistry 2013, ISBN 978-1-84973-670-1 , pp. 982 f.
Individual evidence
- ^ Q. Alan Xu, Timothy L. Madden: Analytical Methods for Therapeutic Drug Monitoring and Toxicology . John Wiley & Sons, 2011, ISBN 0-470-92279-6 , pp. 267 ( limited preview in Google Book search).
- ↑ a b c d e entry on cetobemidone. In: Römpp Online . Georg Thieme Verlag, accessed on July 3, 2016.
- ^ J. Elks: The Dictionary of Drugs: Chemical Data, Chemical Data, Structures and Bibliographies . Springer, 2014, ISBN 978-1-4757-2085-3 , pp. 467 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ K. Hardtke et al. (Ed.): Commentary on the European Pharmacopoeia Ph. Eur. 7.0, ketobemidine hydrochloride. Loose-leaf collection, 23rd delivery 2011, Wissenschaftliche Verlagsgesellschaft Stuttgart.
- ↑ Eberhard Klaschik : Pain therapy and symptom control in palliative medicine. In: Stein Husebø , Eberhard Klaschik (ed.): Palliative medicine. 5th edition, Springer, Heidelberg 2009, ISBN 3-642-01548-4 , pp. 207-313, here: p. 234.