Acetorphine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
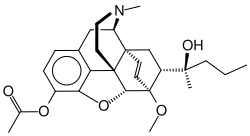
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Acetorphine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 27 H 35 NO 5 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| Drug class | ||||||||||||||||
| Mechanism of action | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 453.57 g · mol -1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Acetorphin is a strong opioid analgesic , up to 8,700 times more pain-relieving than morphine and 2.7 times more potent than etorphine (propylorvinol) under the same test conditions .
Acetorphine is the acetic acid ester of etorphine. Etorphine is used as a very potent pain reliever ( analgesic ) and narcotic ( anesthetic ) in veterinary medicine under the trade name Immobilon , primarily for the immobilization ( sedation ) of large animals such as elephants, giraffes and rhinos.
history
Acetorphin was developed in the early 1960s by the Reckitt research group, who also developed Etorphin. Acetorphine, like etorphine and dihydroetorphine, was developed with the same intention of obtaining opioid analgesics without undesirable side effects ( development of tolerance , development of addiction ). The high potency of these compounds was neither the focus of development nor was it expected, but then aroused interest because the substances were suitable as strong sedatives for immobilizing large animals in veterinary medicine. Despite some advantages over etorphine, such as the lower toxic side effects in giraffes, acetorphine never became popular as a veterinary drug and etorphine (along with other sedatives such as carfentanil , thiafentanil (A-3080) and azaperone) are considered the drug of choice for such uses.
Others
The 2.7 -fold increase in the potency of etorphine (n-propylorvinol) through esterification of the phenolic OH group is contrary to the observation that with all other etorphine analogues an analogous esterification leads to a sometimes strong reduction in potency. For example, as a free phenol, isoamylorvinol is 9200 times more potent than morphine and almost 2.9 times more potent than etorphine. In contrast, isoamylorvinol-3-acetate has a 7 times lower potency than isoamylorvinol and only 0.4 times the etorphine potency or 1300 times the morphine potency. In the case of morphine, acetylation (or general esterification) of the phenolic OH group does not generally change the potency.
At the beginning of the 1960s, at that time without any knowledge of opioid receptors and the biochemical mechanisms of action of opioids, the aim of the development was to create substances through structural modification which, with a strong analgesic effect, were to be characterized by a lower level of addiction compared to the previously known opioids. By chance, compounds (etorphine, dihydroetorphine) were obtained which met these expectations and also had an extremely high, previously unknown potency. Because of their high potency, at that time these compounds were considered completely unsuitable for human medicine and they were not examined in sufficient detail (especially when administered chronically). Therefore, the comparatively low physical addiction potential of etorphine and dihydroetorphine was not recognized at the time. This property was only discovered in the late 1970s. From then on, etorphine and dihydroetorphine were used in experimental pharmacology to investigate the biochemical mechanisms of opioid dependence.
Legal status
In Germany, acetorphin is subject to the Narcotics Act and is listed as a non-marketable narcotic in Appendix I of the Act.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Bentley, KW; Hardy, DG (1967). Novel analgesics and molecular rearrangements in the morphine-thebaine group. 3. Alcohols of the 6,14-endo-ethenotetrahydrooripavine series and derived analogs of N-allyl normorphine and norcodeine . Journal of the American Chemical Society 89 (13): 3281-3292, doi : 10.1021 / ja00989a032 .
- ^ Robert E. Lister, Structure-activity requirements in some novel thebaine-derived analgesics. J. Pharm. Pharmacol. 16: 1964, 364-366.
- ↑ Mao Huang, Bo-Yi Qin Physical dependence of Dihydroetorphine in Mice and Monkeys. Acta Pharmaceutica Sinica 3 (2): 1982, 81-84.
- ↑ BY Qin, M. Huang, YC Zhang, H. Miao Comparison of the dependence potential of Dihydroetorphine, Etorphine an Morphine , Regulatory Peptides 54: 1994, 237-238.
- ↑ M. Huang, DX Wang, BY Qin Dihydroetorphine, a potent opioid with low dependence potential , Regulatory Peptides 53: 1994, 81-82.
- ↑ BY Qin, DX Wang, M. Huang The application of Dihydroetorphine to detoxification of Heroin addicts Regulatory Peptides 53: Suppl. 1 , 1994, 293-294.