Thiafentanil
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
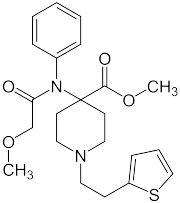
|
||||||||||
| General | ||||||||||
| Surname | Thiafentanil | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 22 H 28 N 2 O 4 S | |||||||||
| Brief description |
white powder (thiafentanil oxalate) |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 416.53 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
190–192 ° C (thiafentanil oxalate) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Thiafentanil (A-3080) is a highly potent and fast acting opioid that is used to anesthetize wild animals using arrow syringes. It is very similar to carfentanyl or etorphine , but differs from them in that it has a faster onset of action, a significantly shorter duration of action and a lower respiratory-depressive potency at narcotically equivalent doses.
Thiafentanil (A-3080) is an analogue of thiofentanil (R 31826), where the propionyl residue has been replaced by a methoxyacetyl residue. It was patented in 1986 along with a whole series of analogous alkoxyalkanamido, furamido and thenamido derivatives. Thiafentanil has been used in veterinary medicine for stunning larger animals, alongside carfentanyl (Wildnil) and etorphin (M99, Immobilon), since the late 1980s .
properties
Thiafentanil differs from carfentanyl in that it has a significantly faster onset of action and a significantly shorter duration of action. The animals awaken from anesthesia 2–5 times faster , depending on the dose and species, within 27–106 minutes after anesthesia . The short duration of action is an advantage compared to carfentanyl because the risk of renarcosis is much lower when using an antidote . In addition, the respiratory depressive potency is lower at equivalent narcotic doses than with carfentanyl or etorphine. The therapeutic index is about 4 times higher compared to carfentanyl. Tachycardia and tachypnea or bradycardia and bradypnea , hypo- or hypertension , respiratory depression, cyanosis , poikilothermia and overheating can occur as undesirable and serious side effects during the course of anesthesia , which is why the duration of anesthesia should be kept as short as possible. The anesthesia is therefore usually canceled with an antidote ( naltrexone or diprenorphine ). In the case of naltrexone, 10-100 times the amount by weight (based on thiafentanil) is used. Because of the short duration of action of thiafentanil, renarcotization is very unlikely. This is a decisive advantage compared to carfentanyl, in which after antagonization, depending on the antagonist and dose, renarcotization can occur after a few hours.
The information on the narcotic potency of thiafentanil fluctuates in the literature. Depending on the study, the carfentanyl potency is 0.63 times or even a slightly higher carfentanyl potency up to 2 times the potency of etorphine. For example, with Impalas, the effective dose which leads to immobilization in 90% of the animals, with thiafentanil at an ED 90 = 80.7 µg / kg and with carfentanyl at an ED 90 = 68.8 µg / kg. The ED 50 value in mice is 0.7 μg / kg (intravenous, hot plate).
Thiafentanil can be used as a single substance, but is usually combined with other substances (usually xylazine , medetomidine and / or ketamine ) that allow the dose to be reduced and at the same time minimize the side effects.
When thiafentanil is used alone as an anesthetic, it triggers the strong muscle rigidity (immobilization) typical of highly potent and fast-acting opioids, which makes working on anesthetized animals difficult. Intubation can also be very difficult in this condition. In combination with alpha agonists (xylazine, medetomidine), muscle rigidity is reduced via central mechanisms.
Others
The replacement of the thiophene residue by a phenyl residue leads to a reduction in the analgesic potency by about four-fold. Methyl {1- (2-phenylethyl) -4- [ N -phenyl-methoxyacetamido] piperidine-4-carboxylate} has an ED 50 value of 3 µg / kg in mice (hot plate, intravenous) (thiafentanil for comparison 0, 7 µg / kg).
Thiafentanil should only be used by appropriately trained personnel and taking appropriate precautions. Corresponding antidotes ( naloxone , naltrexone, diprenorphine) must be available at all times. Injury with contaminated needles or even spraying with injection solution can cause serious poisoning. An accident with carfentanyl has been described in the literature, in which a veterinarian accidentally splashed his face with an injection solution of 1.5 mg carfentanyl and 50 mg xylazine and some of it got into his eyes and mouth. Despite immediate rinsing of the mouth and rinsing of the eyes and face, symptoms of intoxication were evident within 2 minutes.
Individual evidence
- ↑ a b c Wisconsin Department of Safety and Professional Services CONTROLLED SUBSTANCES BOARD ( Memento of the original from December 4, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c Patent US4584303 : N-aryl-N- (4-piperidinyl) amides and pharmaceutical compositions and method employing such compounds. Published on April 22, 1986 , inventor: KH Deutsche, B.-S. Huang, LV Kudzma, NL Lalinde, RC Terrell.
- ^ R. Eric Miller, Murray E Fowler: Fowler's Zoo and Wild Animal Medicine Current Therapy, Volume 7 . Elsevier Health Sciences, London 2011, ISBN 978-1-4377-1985-7 .
- ↑ a b D.L. Janssen, GE Swan, JP Raath, SW McJames, JL Allen, V. de Vos, KE Williams, JM Anderson, TH Stanley: Immobilization and Physiologic Effects of the Narcotic A-3080 in Impala (Aepyceros melampus) . In: J. Zoo Wildlife Medicine . tape 24 , 1993, pp. 11-18 , JSTOR : 20460308 .
- ↑ A. Cushing, BVSc. and M. McClean, DVM: Use of Thiafentanil-Medetomidine for the Induction of Anesthesia in Emus (Dromaius novaehollandiae) within a Wild Animal Park . In: J. Zoo Wildlife Med. Volume 41 , 2010, p. 234-241 , doi : 10.1638 / 2009-0143R1.1 .
- ↑ Lisa L. Wolfe, William R. Lance, and Michael W. Miller: Immobilization of mule Deer with Thiafentanil (A-3080) or with Thiafentanil plus Xylazine. In: J. Wildlife Diseases . tape 40 , 2004, pp. 282-287 , doi : 10.7589 / 0090-3558-40.2.282 .
- ↑ DV Cooper, D. Grobler, M. Bush, D. Jessup, W. Lance: Anaesthesia of nyala (Tragelaphus angasi) with a combination of thiafentanil (A3080), medetomidine and ketamine. In: JS Afr. Vet. Assoc. tape 76 , 2005, pp. 18-21 , doi : 10.4102 / jsava.v76i1.388 .
- ↑ D. Grobler, M. Bush, D. Jessup, W. Lance: Anesthesia of gemsbok (Oryx gazella) with a combination of A3080, medetomidine and ketamine . In: Journal of the South African Veterinary Association . tape 72 , no. 2 , September 2001, ISSN 2224-9435 , p. 81-83 , doi : 10.4102 / jsava.v72i2.622 .
- ^ Antony V. George, Jenny J. Lu, Matthew V. Pisano, Jessica Metz, Timothy B. Erickson: Carfentanil — an ultra potent opioid . In: The American Journal of Emergency Medicine . tape 28 , no. 4 , May 2010, p. 530-532 , doi : 10.1016 / j.ajem.2010.03.003 .