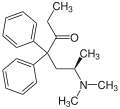
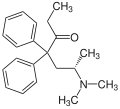
Methadone
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
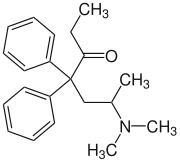
|
|||||||||||||
| Structure without stereochemistry | |||||||||||||
| General | |||||||||||||
| Non-proprietary name | Methadone | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 21 H 27 NO | ||||||||||||
| Brief description |
White to almost white, crystalline powder (L-polamidon hydrochloride) |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| properties | |||||||||||||
| Molar mass | 309.45 g · mol -1 (methadone) | ||||||||||||
| Melting point | |||||||||||||
| pK s value |
8.94 |
||||||||||||
| solubility | |||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Methadone is a fully synthetic opioid with a strong analgesic effect. Methadone is a pure agonist on the μ-opioid receptor and probably on the δ-opioid receptor. It has proven its effectiveness as a heroin substitute in the context of substitution programs and was therefore included in the list of indispensable drugs of the World Health Organization by the World Health Organization (WHO) in 2005.
history
The substance, later named methadone, was developed in 1937 by Max Bockmühl and Gustav Ehrhart , two employees of the Hoechster Farbwerke belonging to the IG Farben group , with the synthesis number VA 10820 and a patent applied for in 1938. The analgesic effect of VA 10820 was first established in 1942 in a small clinical study. It was not until 1945 that Otto Schaumann , or, independently of him, Charles C. Scott and KK Chen, both employees at Lilly Research Laboratories. by Eli Lilly , definitely proven. After World War II , VA 10820 came to the United States via patent and regulatory expropriation. 1947 received VA 10820 the generic name methadone or in the US Methadone . In the same year, Eli Lilly launched a racemic methadone under the brand name Dolophine . Any foreign company could acquire the manufacturing rights. Methadone was later marketed worldwide under various brand names. In January 1949, after the dissolution of IG Farben, Hoechst AG was able to bring methadone monochiral under the name L-Polamidon ( levomethadone ) to the market as a strong pain reliever .
Methadone has been used as a substitute drug for heroin addiction since the 1960s (first in the USA) , whereby in the first few years only very high doses were used in highly structured programs with the aim of permanent substitution - this because opioid addiction was seen as a metabolic disorder, which is to be treated like other metabolic diseases . In Germany, the substitution method with dihydrocodeine was introduced by the Kiel doctor Gorm Grimm .
chemistry
Extraction and presentation
Methadone is produced fully synthetically, in contrast to z. B. Heroin, which is semi-synthetically made from the natural opium alkaloid morphine . In chemical and structural terms, methadone differs significantly from morphine and heroin. The technical synthesis of racemic methadone is quite simple and is based on diphenylacetonitrile which is easily obtained by Kolbe nitrile synthesis .
Stereochemistry
Methadone is chiral , so it is usually a 1: 1 mixture ( racemate ) of two mirror-image molecules ( enantiomers ). In contrast to the left-turning levomethadone , the right-turning dextromethadone is a potent antitussive (cough blocker), but has almost no analgesic potency . It follows that Levomethadon ( L -Polamidon) is twice as strong analgesic activity as the rac -methadone and Methadone ( L -Polamidon) therefore against rac is only half as high dosing -methadone. In Germany, rac methadone ( Methaddict ® tablets or as a basic substance) and levomethadone for opioid substitution (e.g. for heroin ) or as a strong pain reliever ( analgesic ) can be prescribed (according to Appendix III - BtMG BTM prescription ) and only through pharmacies available as a patient.
The racemic mixture can be separated using diastereomeric salts with L - (+) - tartaric acid .
The pure enantiomers of the free base melt at 100 ° C. The racemate exists as a racemic mixture with a eutectic melting point of 77 ° C. Enantiomerically pure methadone hydrochloride has a melting point of 248 ° C. In the case of the 1: 1 mixture of the enantiomeric hydrochlorides, a racemic compound with a melting point of 237 ° C is formed, the eutectic melting points with the enantiomers in the phase diagram at 233 ° C with compositions of 0.29 / 0.71 and 0.71 / 0.29 show.
Analytics
Methadone responds as a CH-acidic compound to the Zimmermann / Janovski reaction . After adding 1,3-dinitrobenzene and potassium hydroxide in methanol, it forms a red-violet colored Meisenheimer complex .
The reliable qualitative and quantitative determination of methadone takes place after appropriate sample preparation by coupling the HPLC with the mass spectrometry . Blood or saliva, hair, bone material and waste water samples can be used as test material. or in the aerosol of breathing air samples The use of gas chromatography with mass spectrometry coupling has also been described for various test items .
pharmacology
Methadone activates the μ- opioid receptor and from a dose of 2.5 mg (in adults) develops its pain-relieving effect, lasting three to four hours, which is 1.5 times that of morphine based on an even single dose . Methadone activates the dopaminergic system in the ventral tegmentum much less than morphine . This is attributed to the fact that, in contrast to morphine, methadone has a weak potency at a receptor complex consisting of the μ-opioid receptor and the galanin receptor type 1. Activation of this receptor heteromer is associated with euphoria.
Methadone dose-dependently inhibits the HERG channel , a voltage-activated, inwardly rectifying potassium channel in heart muscle cells, which leads to a prolongation of the QT time ( see Long QT syndrome ).
Dextromethadone is an NMDA receptor antagonist. This explains its effect on neuropathic pain.
Methadone is mainly broken down via the isoenzyme CYP3A4 to the inactive metabolites 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) and 2-ethyl-5-methyl-3,3-diphenylpyraline (EMDP). To a lesser extent, 2B6, 2C8, 2C18, 2C19 and 2D6 play a role. Simultaneous use of drugs that block this breakdown pathway leads to an increase in the plasma concentration of methadone, as does the use of drugs that are strongly bound to plasma proteins, since 85-90% of methadone is bound to these proteins . Conversely, cocaine can decrease methadone plasma levels. Tabular lists of the interactions of methadone with other drugs are available.
Effects
Medicinal effects
As an opioid, methadone has the same profile of effects and side effects and thus essentially the same risk potential as other opioids (exception: buprenorphine ). However, it does not create a kick (that sudden, intense feeling of well-being that leads to the development of an addiction).
Methadone is used primarily in a wide variety of dispensing programs for heroin addicts . In this regard, methadone administration is one of the most effective therapies of all. However, it must also be emphasized that a large part of the intended effects of methadone is due to the fact that it is offered in special, structured programs . For most of the participants, a single daily dose leads to the desired stabilization, whereby a Cochrane study was able to prove at least beyond doubt that methadone can keep patients in care and that there is less heroin consumption. It should be noted here that doses of up to 40 mg (which the patient himself described as sufficient) can easily be "overcome" by commercial street heroin. An opioid blockade effect can only be achieved from doses of 60 mg: This means that the additional consumption of commercially available opioids such as morphine , heroin or hydromorphone does not cause any narcotic (and euphoric) effects.
Some substituted people break down methadone more quickly (so-called " fast metabolizers "), so that withdrawal symptoms can occur at the end of a 24-hour dose interval . U. must be dispensed in two daily doses. If the goal of treatment is abstinence from opioids , the withdrawal symptoms can be kept bearable by slowly tapering off. The heroin withdrawal itself can also be absorbed and completed with methadone (e.g. during hospital and prison stays). A slow tapering off of methadone can take several months and from a certain residual amount it can lead to unpleasant withdrawal symptoms.
Investigated effect in cancer therapy
The European Organization for palliative care ( "European Association for Palliative Care", EPAC) recommends methadone as a painkiller accompany the cancer treatment if patients do not benefit from morphine or suffer from side effects.
A possible intensifying effect of methadone on cytostatics as a "cancer agent" (such as doxorubicin ) is the subject of initial studies, initiated by Claudia Friesen at the Institute for Forensic Medicine at Ulm University . From in vitro studies it has been postulated that methadone in a variety of solid tumors and leukemia a cell death could cause. Activation of the opioid receptor via inhibitory G i proteins, presumably induced by methadone, is said to lead to a downregulation of cAMP . In leukemia cells this leads to apoptosis, since caspases are activated. In addition, methadone is said to disrupt intracellular calcium metabolism, causing a morphological and functional change in the mitochondria . However, it is not exactly known which anti-tumor mechanism is underlying. The cell line experiments also used methadone concentrations that were higher than the therapeutic levels. Further experiments were carried out on severely immunodeficient nude mice . There an effect was shown with a methadone dose of 240 mg. The lethal daily dose in unadjusted people is 40 to 60 mg, but mice metabolize opioids more quickly than humans.
In a retrospective study, 76 out of a collective of 938 cancer patients were identified who had been switched from another opioid to methadone because of pain. These were compared with 88 patients who had been switched to another opioid in a similar situation. There were no differences with regard to overall survival. Data from another study with 52 cancer patients (head and neck tumors) also showed no evidence of an advantage of methadone in terms of survival compared to another opioid (here fentanyl ).
Methadone is used in cancer therapy as a classic pain reliever, application studies have so far not proven any anti-tumor effects with methadone (or other opioids). The German Society for Hematology and Medical Oncology and the German Society for Neurology have doubts about the effectiveness of methadone in cancer therapy and warn of unrealistic expectations that may arise after the publication of a single study from 2017 (with 27 cancer patients with glioblastomas of different stages without naming a control group ) could have arisen. This study also only analyzed the tolerability of methadone administration retrospectively. The spokesman on the board of the Neuro-Oncological Working Group (NOA), Wolfgang Wick , considers the database to be insufficient as an approach for a possible therapy. The University of Ulm , at whose institute for forensic medicine the first scientific findings were obtained, expressly distances itself and does not consider the uncritical use of methadone outside of clinical studies to be justified. Austrian societies such as the OeGHO or the ÖSG (Austrian pain society ) also joined in.
2017 wrote the Medical Center for Quality in Medicine on behalf of the physicians' Confederation and the German Medical Association , a patient information about methadone in cancer therapy . Also in 2017, funding for a study was applied for at the German Cancer Aid to check the effect in cancer therapy. In 2019, a clinical trial in patients with metastatic colon cancer was approved and is scheduled to start in 2020 ("MEFOX study"). The first results are expected for 2022. In cell culture experiments, however, it has already been shown that R - (-) - methadone ( levomethadone ) is not effective in glioblastomas, either alone or in combination with the chemotherapy drug temozolomide . A possible reason for the lack of effectiveness is the lack of specific docking sites (which could not be shown in any other study), so that methadone could not develop any anti-tumor effects in the glioblastoma cell cultures examined. A group of researchers has shown 2018 that D - / L induced -methadone with or without temozolomide in glioblastoma cell lines only at high doses cell death - in concentrations which are clinically feasible showed D - / L -methadone in vitro , however no effect. A study carried out in 2019 confirmed previous cell culture experiments: In glioblastoma cells taken from cancer patients, which this time have receptors (with healthy cells for comparison), methadone only has an effect in plasma concentrations at which it would be fatal for humans. However, effective brain tissue concentrations can be achieved.
Ulm University holds various patents for the use of methadone and opioids in cancer patients, in which Friesians and others are named as inventors.
Side effects
An assessment of the undesirable effects of methadone depends on several prerequisites. Firstly, a person with an opiate addiction that has existed for several years with regard to the effects and side effects of an opiate substitute can hardly be compared with a person with little drug experience. Second, indicated side effects of the drug can be understood as symptoms of illnesses that already existed at the time of drug use or were caused by drug use and were not appropriately perceived or taken into account at that time. Thirdly, the specifics of the drug culture with its specific points of view must also be taken into account. In fact, many undesirable effects can be explained as a result of an unsuitable drug dose at the beginning of the substitution treatment.
The desired euphoria is increasingly difficult to achieve due to the development of tolerance, despite higher heroin doses. The fact that those substituted with high doses of methadone and with long enough treatment rarely complain of adverse effects - such as drowsiness and drowsiness - than those with low doses of methadone (3.9% compared to 4.3%) is probably due to this development of tolerance.
Side effects can occur with methadone doses that exceed the pre-existing tolerance to opioids due to previous consumption. These are tiredness, sleep disorders , drowsiness , nausea , vomiting , profuse sweating, edema (fluid retention) in the legs, urinary retention and constipation (constipation). They usually go away as tolerance develops or the dose is reduced. Sleep and sexual disorders last the longest (with a substitution period of three years, this is still around 20 percent). Up to 50 percent of those who are substituted complain of increased sweating ( hyperhidrosis ) for longer .
Use during pregnancy and breastfeeding: Methaddict tablets have an effect on the fetus when taken during pregnancy. It is therefore recommended to gradually end the methadone substitution before birth . If tapering off is not possible, the newborn must be withdrawn in an intensive care unit. There were ocular abnormalities , neurological findings with hearing impairments , mental and motor development delays and increased incidence of otitis media observed. Since methadone is excreted in breast milk , a doctor must decide on a case-by-case basis whether the mother can breastfeed her child.
QT time extension
A prolongation of the QT time in the ECG is a risk factor for the occurrence of cardiac arrhythmias and especially of potentially life-threatening torsade de pointes (TdP). A number of factors have been identified that increase the probability of a QT time prolongation and subsequent TdP namely, female gender, hypokalaemia , decreased serum magnesium levels, known history of drug interactions , pre-existing heart problems, undetected congenital long QT syndrome (LQTS), and predisposing DNA polymorphism . So far, however, no information can be given on the significance of these factors for methadone-substituted people. Nevertheless, they must be taken into account in substitution therapy. About 2% of patients receiving methadone develop a prolonged QT time, of which about 2% develop torsades de pointes. In 2009, guidelines for QT time screening for methadone substitution were published in the US. QT extensions and torsade de pointes episodes were also originally the reason for the suspension of marketing for LAAM .
The QT time prolongation is mainly mediated via dextromethadone, a QT time prolongation therefore only occurs when substituting with the racemate. A QT time lengthening induced by the racemate can be reversed by changing the substitution to levomethadone (L-polamidone). No clinically relevant changes in the QT time are detectable for doses below 100 mg per day.
Methadone administration is considered safe as long as the possibility of a QT time lengthening is taken into account, checked by means of ECG controls before therapy, one month after the start of therapy and then at annual intervals, the patient is informed and appropriate consequences are drawn from the results. As a result, these guidelines do not recommend a switch to another substitution agent, since slow-release morphines, which do not cause a change in the QT time, are not permitted in substitution programs in the USA. In Austria, it is possible to switch to these retarded morphines.
Intoxication
Signs of an overdose of opioids are constricted pupils up to the size of the head of a pin ( miosis ), severe respiratory depression and impaired consciousness up to coma (as a so-called opioid triad), drop in blood pressure with tachycardia , hypothermia and weakened reflexes up to areflexia . Less than a milligram per kilogram of body weight can be fatal in people without opioid tolerance. This means that the lethal dose for a young child can be less than 10 mg and for an adult 40–50 mg. When stopped on methadone, deaths in the first two weeks of treatment were associated with a dose range of 25-100 mg, with most occurring at doses of 40-60 mg. Whenever possible, patients should therefore be checked for signs of overdose (or persistent withdrawal symptoms) at the time of first peak effect (three to four hours after first ingestion). To treat an overdose, antagonistic drugs such as B. naloxone or naltrexone are available, whereby the duration of action of the former is significantly shorter (approx. 1 hour) than that of the active ingredient methadone or levomethadone (up to 48 hours respiratory depressive effect) and must therefore be re-dosed several times.
Opioid addicts with regular use are less susceptible to intoxication than occasional users or addicts after opioid withdrawal .
application
Methadone is available in drop or tablet form, in Austria only as a syrup. It has a comparatively high oral bioavailability of around 80%. For ingestion, it is usually diluted or colored with sugar syrup, juice or water, in order to prevent improper intravenous consumption when a take-home is given. In Germany, the on-site administration of liquid preparations has prevailed. This has the advantage of being able to reduce the dose slowly in very small (up to dropwise) steps, which helps to avoid withdrawal symptoms. This also prevents patients from spitting out tablets that have not been swallowed in order to inject or sell them later, as often happens with Subutex (active ingredient: buprenorphine ). Methadone tablets can be taken directly without prior dissolution.
In Germany, levomethadone is used as a highly potent pain reliever for the treatment of severe acute and chronic pain. The drug is available as a solution (at a concentration of 5 mg / ml) for oral use and in ampoules for injection. A retrospective cohort study with over 30,000 evaluated patient data has shown that the risk of death was significantly higher with methadone than with morphine - even at low doses. In the opinion of the authors, methadone should therefore not be used as the first treatment option for non-tumor-related pain.
Abuse and disclosure
There is an illegal market for methadone, as some substitutes resell the drug after it has been dispensed by pharmacies. Methadone-related deaths hit four-digit case numbers in the US since the turn of the millennium. In deaths with prescription opiate painkillers, it was often found that the deceased had obtained them without a prescription and that the consumption was in combination with other, illegal substances.
In Austria and some German substitution practices, this is one of the reasons why the patient must personally pick up his substitution drug every day. Exceptions are regulated in Austria with § 23e of the legal provision for the Narcotics Ordinance.
In Austria, the passing on of the substitution drug methadone is a criminal offense under the Narcotics Act . Exclusion from substitution treatment can take place through, among others
- The use of other substances that endanger the substitution treatment or the state of health,
- passing on or intravenous use of the substitution drug,
- the illegal trade in narcotic drugs or drugs containing drugs and
- the improper use of recipes.
Trade names
Monopreparations : Heptadon (A), Ketalgin (CH), Methaddict, Mephenon (FRA), (L-) Polamidon, Eptadone (D), Methaliq (D) and as a generic (CH)
See also
literature
- Hans V. Happel, Frank Männike: Survival with methadone. For an alternative drug policy . Concrete literature, Hamburg 1992, ISBN 3-89458-116-6 .
- U. Honegger, A. Seidenberg: Methadone, heroin and other opioids: Medical manual for outpatient opioid-assisted treatment . Huber, Bern a. a. 1998, ISBN 3-456-82908-6 .
- R. Gerlach, H. Stöver: From taboo to normality - 20 years of substitution in Germany . Lambertus, Freiburg i.Br. 2005, ISBN 3-7841-1605-1 .
- Eberhard Klaschik : Pain therapy and symptom control in palliative medicine. In: Stein Husebø , Eberhard Klaschik (ed.): Palliative medicine. 5th edition, Springer, Heidelberg 2009, ISBN 3-642-01548-4 , pp. 207-313, here: pp. 234 and 248 f.
Web links
- Methadone / substitution treatment index
- Methadone . In: Erowid . (English)
- Methadone in oncology: “straw function” without evidence . Deutsches Ärzteblatt 2017; 114 (33-34): A-1530 / B-1298 / C-1269
- Methadone for cancer? . Arznei-Telegram, June 2, 2017
- Does methadone help against cancer? In: quarks.de . October 29, 2019, accessed January 3, 2019 .
Individual evidence
- ↑ a b European Pharmacopoeia Commission (Ed.): European Pharmacopoeia 5th Edition . tape 5.0 - 5.7 , 2006.
- ↑ a b c d e M Kuhnert-Brandstätter, L. Friedl: Contribution to thermal analysis and the polymorphism of optical antipodes: pantolactone, methadone and usnic acid. Microchim. Acta 1979, Vol. 72, pp. 97-110, doi: 10.1007 / BF01198052 .
- ↑ a b c d Entry on methadone in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b data sheet (±) -Methadone hydrochloride from Sigma-Aldrich , accessed on April 10, 2011 ( PDF ).
- ↑ a b c d e Friedemann Nauck et al .: Methadone as anticancer treatment: hype, hope, or hazard? In: Wiener Medical Wochenschrift . tape 168 , no. 7-8 , May 1, 2018, pp. 159–167 , doi : 10.1007 / s10354-018-0623-5 ( springer.com [accessed January 4, 2019]).
- ^ A b R. P. Mattick et al .: Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. (PDF; 369 kB) Cochrane Drugs and Alcohol Group, 2009.
- ^ WHO Model List of Essential Medicines . (PDF; 442 kB) accessed on September 20, 2012.
- ^ Foundation for a Drug-Free World: The Truth About Painkillers. Drug-Free World, p. 16. ( limited preview in Google Book search)
- ↑ M. Bockmühl, G. Ehrhart: Process for the preparation of basic esters. German Reich patent No. 711069, filing date: September 11, 1938, publication: September 25, 1941.
- ↑ PO Wolff: On pethidine and methadone derivatives. In: Bulletin of the World Health Organization. Volume 2, Number 2, 1949, pp. 193-204. PMID 15409516 . PMC 2553950 (free full text).
- ^ CC Scott and KK Chen: The action of 1,1-diphenyl-1- (dimethylaminoisopropyl) -butanone-2, a potent analgesic agent. In: Federation proceedings. Volume 5, Number 1, 1946, p. 201. PMID 20983210 .
- ^ EM Stoya: M for methadone. ( Memento from September 9, 2012 in the web archive archive.today ) In: The PTA in the pharmacy. 11, 2011, p. 20.
- ↑ a b c d Nicholas Seivewright, assisted by Mark Parry: Community Treatment of Drug Misuse: More Than Methadone. Cambridge University Press, 2009.
- ↑ A. Ulmer, R. Ullmann: Obituary Gorm Grimm. ( Memento from November 24, 2015 in the Internet Archive ) (PDF; 292 kB) In: Addiction therapy. 9, 2008, pp. 185-186. doi: 10.1055 / s-0028-1102925 .
- ↑ E. Breitmaier: Alkaloids. Teubner-Verlag, 1997, p. 158.
- ↑ Methaddict ® is the patented trade name of Hexal
- ↑ Merck & Co., US 2,644,010,1953.
- ↑ Strand MC, Ramaekers JG, Gjerde H, Mørland J, Vindenes V: Pharmacokinetics of Single Doses of Methadone and Buprenorphine in Blood and Oral Fluid in Healthy Volunteers and Correlation With Effects on Psychomotor and Cognitive Functions. , J Clin Psychopharmacol. 2019 Sep / Oct; 39 (5): 489-493, PMID 31305338
- ↑ Kronstrand R, Forsman M, Roman M: Quantitative analysis of drugs in hair by UHPLC high resolution mass spectrometry. , Forensic Sci Int. 2018 Feb; 283: 9-15, PMID 29241093
- ↑ Vandenbosch M, Somers T, Cuypers E: Distribution of Methadone and Metabolites in Skeletal Tissue. , J Anal Toxicol. 2018 Jul 1; 42 (6): 400-408, PMID 29490025
- ↑ Bade R, White JM, Gerber C: Qualitative and quantitative temporal analysis of licit and illicit drugs in wastewater in Australia using liquid chromatography coupled to mass spectrometry. , Anal Bioanal Chem. 2018 Jan; 410 (2): 529-542, PMID 29214532
- ↑ Ullah S, Sandqvist S, Beck O .: A liquid chromatography and tandem mass spectrometry method to determine 28 non-volatile drugs of abuse in exhaled breath. , J Pharm Biomed Anal. 2018 Jan 30; 148: 251-258, PMID 29059614
- ↑ Ribeiro A, Prata M, Vaz C, Rosado T, Restolho J, Barroso M, Araújo ARTS, Gallardo E: Determination of methadone and EDDP in oral fluid using the dried saliva spots sampling approach and gas chromatography-tandem mass spectrometry. , Anal Bioanal Chem. 2019 Apr; 411 (10): 2177-2187, PMID 30824966
- ↑ Hsu YC, Chen BG, Yang SC, Wang YS, Huang SP, Huang MH, Chen TJ, Liu HC, Lin DL, Liu RH, Jones AW: Methadone concentrations in blood, plasma, and oral fluid determined by isotope-dilution gas chromatography-mass spectrometry. , Anal Bioanal Chem. 2013 May; 405 (12): 3921-8, PMID 23090648
- ↑ Eberhard Klaschik : Pain therapy and symptom control in palliative medicine. 2009, p. 234.
- ↑ Cai NS, Quiroz C, Bonaventura J, et al .: Opioid-galanin receptor heteromers mediate the dopaminergic effects of opioids . In: J. Clin. Invest. tape 129 , issue 7, 2019, pp. 2730-2744 , doi : 10.1172 / JCI126912 , PMID 30913037 , PMC 6597217 (free full text) - ( jci.org ).
- ↑ Yutaka Oda, Evan D. Kharasch: Metabolism of Methadone andlevo-α-Acetylmethadol (LAAM) by Human Intestinal Cytochrome P450 3A4 (CYP3A4): Potential Contribution of Intestinal Metabolism to Presystemic Clearance and Bioactivation. In: J Pharmacol Exp Ther . 298 (3), Sep 2001, pp. 1021-1032. PMID 11504799 .
- ↑ a b Richard P. Mattick et al .: Pharmacotherapies for the Treatment of Opioid Dependence: Efficacy, Cost-Effectiveness and Implementation Guidelines . Informa Healthcare, 2009, ISBN 978-1-84184-400-8 .
- ^ Stewart B. et al .: When "Enough" is not Enough: New Perspectives on Optimal Methadone Maintenance Dose. ( Memento from April 15, 2011 in the Internet Archive ) (PDF; 95 kB) In: Mount Sinai Journal of Medicine. Volume 67, Number 5 & 6, Oct-Nov 2000, pp 404-411.
- ↑ EF McCance-Katz, P. Jatlow, PM Rainey: Effect of cocaine use on methadone pharmacokinetics in humans. In: Am J Addict. 19 (1), Jan-Feb 2010, pp. 47-52. PMID 20132121 .
- ↑ Elinore F. McCance-Katz: Methadone-Drug Interactions (Medications, illicit drugs, and other substances). ( Memento from October 19, 2013 in the Internet Archive ) (PDF; 675 kB).
- ↑ a b Richard A. Rettig, Adam Yarmolinsky, Institute of Medicine (US). Committee on Federal Regulation of Methadone Treatment: Federal regulation of methadone treatment. 1995.
- ↑ Friesen C (2014) Cell Cycle 13 (10): 1560-70. Opioid receptor activation triggering downregulation of cAMP improves effectiveness of anti-cancer drugs in treatment of glioblastoma.
- ↑ Friesen C et al. (2013) Oncotarget 4 (5): 677-90. Cell death sensitization of leukemia cells by opioid receptor activation
- ↑ S. Wirz et al .: Use of methadone to support oncological therapy? In: The pain . tape 31 , no. 1 , February 1, 2017, p. 2-4 , doi : 10.1007 / s00482-016-0183-9 ( springer.com [accessed January 4, 2019]).
- ^ Marieke HJ van den Beuken-van Everdingen, Sander MJ van Kuijk and Elbert A. Joosten: Response to the article "Overall Survival among Cancer Patients Undergoing Opioid Rotation to Methadone Compared to Other Opioids" . In: Journal of Palliative Medicine . tape 20 , no. 11 , November 2017, p. 1184–1185 , doi : 10.1089 / jpm.2017.0301 , PMID 28731784 .
- ↑ Does methadone help against cancer? In: quarks . October 29, 2019, accessed January 21, 2020 .
- ↑ Akhila Reddy et al .: Overall Survival among Cancer Patients Undergoing Opioid Rotation to Methadone Compared to Other Opioids . In: Journal of Palliative Medicine . tape 20 , no. 6 , June 2017, p. 656-661 , doi : 10.1089 / jpm.2016.0316 , PMID 27997283 .
- ^ Marieke HJ van den Beuken-van Everdingen, Sander MJ van Kuijk and Elbert A. Joosten: Response to the article "Overall Survival among Cancer Patients Undergoing Opioid Rotation to Methadone Compared to Other Opioids" . In: Journal of Palliative Medicine . July 21, 2017, doi : 10.1089 / jpm.2017.0301 , PMID 28731784 .
- ^ Philip J. Wiffen et al .: Opioids for cancer-related pain in children and adolescents . In: The Cochrane Database of Systematic Reviews . tape 7 , July 19, 2017, p. CD012564 , doi : 10.1002 / 14651858.CD012564.pub2 , PMID 28722116 .
- ↑ S. Mercadante, A. Casuccio and L. Calderone: Rapid switching from morphine to methadone in cancer patients with poor response to morphine . In: Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology . tape 17 , no. 10 , October 1999, p. 3307-3312 , doi : 10.1200 / JCO.1999.17.10.3307 , PMID 10506634 .
- ↑ Nina Buschek: Brain tumor: Methadone does not increase the effectiveness of chemotherapy. Retrieved June 23, 2018 .
- ↑ Methadone in cancer patients: doubts about effectiveness and safety. DGHO, accessed August 17, 2017 .
- Jump up ↑ J. Onken et al .: Safety and Tolerance of D, L-Methadone in Combination with Chemotherapy in Patients with Glioma. In: Anticancer Research . Volume 37, number 3, 03 2017, pp. 1227-1235, doi: 10.21873 / anticanres.11438 , PMID 28314286 .
- ↑ plusminus August 16, 2017 daserste.de ( Memento from August 20, 2017 in the Internet Archive )
- ^ Felix Hütten: Methadone - miracle drug against cancer? In: sueddeutsche.de . July 24, 2017 ( sueddeutsche.de [accessed June 23, 2018]).
- ^ Opinion on tumor therapy with methadone . ( uniklinik-ulm.de [accessed on June 23, 2018]).
- ^ Opinion - Methadone in cancer patients - News - oegho.at. Retrieved January 4, 2019 .
- ↑ Methadone in tumor patients Statement by the Austrian Pain Society (ÖSG). Austrian Pain Society, July 10, 2017, accessed on January 4, 2019 .
- ↑ methadone. So far no benefit proven: methadone in cancer treatment . Patient-Information.de, accessed on January 29, 2018.
- ↑ Deutsche Krebshilfe wants to support 5 new centers with 50 million euros. In: Deutsches Ärzteblatt. July 5, 2017, accessed January 29, 2018 .
- ↑ Thomas Burmeister: Does methadone help with cancer? Doctors newspaper , October 28, 2019, accessed January 8, 2020 .
- ↑ MEFOX study: methadone plus chemotherapy for metastatic colon cancer. In: Ulm University Hospital. Retrieved March 28, 2020 .
- ↑ Nina Buschek: Brain tumor: Methadone does not increase the effectiveness of chemotherapy. Retrieved September 30, 2018 .
- ↑ Dr. Bettina Jung, pharmacist: methadone in cell experiments in glioblastoma ineffective . In: DAZ.online . March 1, 2018 ( deutsche-apotheker-zeitung.de [accessed October 2, 2018]).
- ↑ Konstantin Brawanski et al .: Efficacy of D, L-methadone in the treatment of glioblastoma in vitro . In: CNS oncology . tape 7 , no. 3 , July 1, 2018, p. CNS18 , doi : 10.2217 / cns-2018-0006 , PMID 29916277 , PMC 6200059 (free full text).
- ↑ Vera Zylka-Menhorn: Glioblastomas: Leipzig Study - Methadone ineffective in the therapy of brain tumors. Deutsches Ärzteblatt, April 19, 2019, accessed on May 27, 2019 .
- ↑ METHADONE HYPE IN CANCER ... Emotions replace proof of effectiveness. Arznei-Telegram , September 15, 2017, accessed on March 11, 2020 .
- ↑ Douglas Goldsmith et al. Methadone Folklore: Beliefs about Side Effects and Their Impact on Treatment. In: Human Organization. 43, 1984, p. 330, doi: 10.17730 / humo.43.4.64m061v484th3871 .
- ^ Dana E. Hunt, Douglas S. Lipton, Douglas S. Goldsmith, David L. Strug, Barry Spunt: Substance Use & Misuse . Vol. 20, No. 11-12, 1985, pp. 1751-1771, doi: 10.3109 / 10826088509047261
- ↑ Eric C. Strain et al .: Moderate vs. High-Dose Methadone in the Treatment of Opioid Dependence - A Randomized Trial. In: JAMA . 281, 1999, pp. 1000-1005.
- ↑ Ruthard Stachowske , website of the Evangelical University of Dresden, accessed on March 22, 2020.
- ↑ J. Stringer, C. Welsh and A. Tommasello: Methadone-associated QT interval prolongation and torsades de pointes. In: Am J Health Syst Pharm . 66 (9), May 1, 2009, pp. 825-833. PMID 19386945
- ↑ QT time screening for methadone ( Memento August 15, 2009 in the Internet Archive ) - US guideline 2009.
- ↑ Nicolas Ansermot et al .: Substitution of (R, S) -Methadone by (R) -Methadone - Impact on QTc Interval Clinical Guidelines. In: Archives of Internal Medicine . Vol. 170 No. 6, Mar 22, 2010, pp. 529-536.
- ↑ Stallvik et al .: Corrected QT interval during treatment with methadone and buprenorphine - relation to doses and serum concentrations . Drug And Alcohol Dependence, PMID 23084592
- ↑ Mori J. Krantz et al .: Clinical Guidelines - QTc Interval Screening in Methadone Treatment. In: Annals of Internal Medicine . vol. 150 no. 6, 17 Mar 2009, pp. 387-395.
- ↑ Mori J. Krantz et al .: Concerns About Consensus Guidelines for QTc Interval Screening in Methadone Treatment. In: Ann Intern Med. 151, 2009, pp. 218-219. PMID 19652193
- ^ A b E. Freye: Opioids in medicine. 8th edition. Springer, 2010, ISBN 978-3-540-88796-6 .
- ↑ Michael Krausz, Christian Haasen and Dieter Naber: Pharmacotherapy of Addiction. Karger Medical and Scientific Publishers, 2003, ISBN 3-8055-7482-7 .
- ↑ a b Felix Tretter and Max Braun: Addiction medicine compact: Addiction diseases in clinic and practice; with 51 tables. Schattauer Verlag, 2008, ISBN 978-3-7945-2611-6 , p. 213.
- ^ Karlheinz Keppler and Heino Stöver: Prison medicine: Medical care under prison conditions. Georg Thieme Verlag, 2009, ISBN 978-3-13-157001-7 .
- ↑ Eberhard Klaschik : Pain therapy and symptom control in palliative medicine. 2004, p. 234 ( levo-methadone ).
- ^ Wayne A. Ray et al .: Out-of-hospital mortality among patients receiving methadone for noncancer pain . In: JAMA internal medicine . tape 175 , no. 3 , March 2015, p. 420–427 , doi : 10.1001 / jamainternmed.2014.6294 , PMID 25599329 , PMC 4346542 (free full text).
- ^ WR Davis, BD Johnson: Prescription opioid use, misuse, and diversion among street drug users in New York City. In: Drug and alcohol dependence. Volume 92, number 1-3, January 2008, pp. 267-276, doi: 10.1016 / j.drugalcdep.2007.08.008 . PMID 17913395 . PMC 2317747 (free full text).
- ↑ Metha-Spritz-Survey (PDF) accept.org
- ^ National Prescription Drug Threat Assessment . (PDF) National Drug Intelligence Center, USA, April 2009. Pages 23–24. (PDF; 3.1 MB)
- ↑ a b Legal provision for the narcotics ordinance in Austria. Version dated December 16, 2009 (with treatment contract).