Meisenheimer complex
As Meisenheimer complexes are in organic chemistry in the narrow sense adducts of aromatic nitro compounds with nucleophiles called. They are also called Meisenheimer salts.
discovery
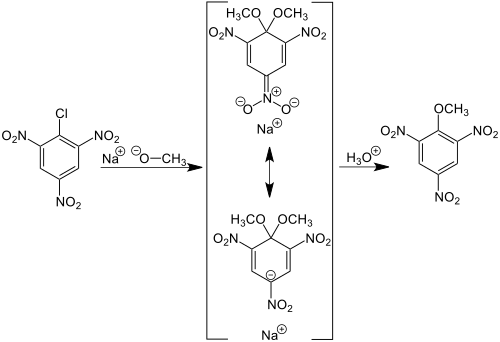
Charles Loring Jackson and WF Boos (Harvard College; USA) discovered at the end of the 19th century that red salts are formed when sodium alcoholates act on the almost colorless 2,4,6-trinitrochlorobenzene ( picryl chloride ). With sodium methoxide / methanol they received a compound for which they proposed the constitutional formula shown in the middle of the formula scheme. Your German name should be 3.5-Dinitro-4.4-dimethoxy-quinolnitrosaures Natrium. If the salt was mixed with dilute mineral acid , 2,4,6-trinitroanisole (right formula) was formed:
Presumably, the researchers had expected that exchanging the chlorine substituent of the picryl chloride for the methoxy radical would result in 2,4,6-trinitroanisole (“methyl picrate”).
At the beginning of the 20th century , Jakob Meisenheimer studied reactions of aromatic nitro compounds in the chemical laboratory of the Royal Academy of Sciences in Munich . Among other things, he produced the suspected alkyl picrates by reacting alkyl iodides with the silver salt of picric acid . In 1902 he announced that the conversion of alkyl picrates with “ alcoholic potash ” results in salts which are noticeable due to their deep red color. Meisenheimer was able to confirm the still valid constitution. In today's language, alkyl picrates are 1-alkoxy-2,4,6-trinitrobenzenes, "alcoholic potash" is a solution of potassium hydroxide in the corresponding alcohol.
Crucial for this realization was that the same salt was formed from methyl picrate and potassium ethoxide as well as from ethyl picrate and potassium methoxide:
The "Meisenheimer salts" decomposed when diluted aqueous sulfuric acid was added. Both ethyl picrate and methyl picrate arose from the "ethoxy-methoxy salt", in accordance with Meisenheimer's and Jackson's constitutional formula.
structure
As far as the structure of the salts is concerned, Jackson and Meisenheimer's proposal came very close to reality. Although we now expect the negative charge to be delocalized (formula below left), Meisenheimer chose the formula of a nitronate . An X-ray structure analysis of the “1,1-dimethoxy complex” showed that the C-4 / NO 2 bond is significantly shorter than that of C-2 or C-6 and nitrous nitrogen.
The bonds between C-2 and C-3 or C-5 and C-6 are shorter than the bonds C-3 / C-4 or C-4 / C-5. This shows that the nitro group in position 4 on the ring takes over most of the negative charge, in the sense of the second boundary structure formula from the left in the figure above. Quantum chemical calculations ( SCF method ) agree well with the result of the X-ray structure analysis.
The intermediates with tetragonal (sp 3 -hybridized) carbon atoms occurring in the S N Ar mechanism are sometimes referred to as Meisenheimer complexes in the broader sense. According to the history of the discovery, it would be justified to mention the American chemist as well; H. to speak of Jackson-Meisenheimer complexes .
meaning
The discovery stimulated further investigations into benzenoid compounds with acceptor substituents. They finally culminated in the finding that compounds of this type can react according to a nucleophilic association / dissociation mechanism (also called addition-elimination mechanism - S N Ar), as discussed in the article Nucleophilic aromatic substitution . So z. B. implement 2,4,6-trinitrochlorobenzene with numerous nucleophiles.
Janovsky reaction
The Janovsky reaction is a detection reaction for nitroaromatics , in which a colored complex is formed by adding aldehydes or ketones . It was discovered and mentioned in 1886 by Jaroslav Janovsky and L. Erb. The ketone is deprotonated by a strong base (e.g. potassium hydroxide solution ). The lone pair of electrons on the 1,3-dinitro aromatic now attacks .
The figure shows the postulated course of the reaction using the example of acetone and 1,3-dinitrobenzene :
Individual evidence
- ↑ CL Jackson and WF Boos, American Chemical Journal 20, pp. 444-447 (1898).
- ↑ C. Loring Jackson and WF Boos, On the Colored Compounds Obtained from Sodic Alcoholates and Picrylchloride, Proceedings of the American Academy of Arts and Sciences Vol. 33, No. 10 (Jan. 1898), pp. 173-182. JSTOR 20020770
- ↑ Beilstein's Handbook of Organic Chemistry, Hauptwerk, Vol. 5, p. 273.
- ↑ Jakob Meisenheimer: About reactions of aromatic nitro bodies . In: Justus Liebig's Annals of Chemistry . tape 323 , no. 2 , 1902, pp. 205-246 , doi : 10.1002 / jlac.19023230205 . especially pp. 241-246.
- ↑ H. Ueda, N. Sakabe, J. Tanaka, A. Furusaki, Bull. Chem. Soc. Japan 41, 2866 (1968).
- ↑ H. Hosoya, S. Hosoya, S. Nagakura, Theoret. Chim. Acta 12, 117 (1968).
- ↑ Siegfried Hauptmann , Organic Chemistry, 1st edition, p. 302, Verlag Harri Deutsch, Thun-Frankfurt a. M., 1985. ISBN 3-87144-902-4 .
- ↑ a b Zerong Wang: Comprehensive Organic Name Reactions and Reagents, 3 Volume Set . John Wiley & Sons, Hoboken, New Jersey 2009, ISBN 978-0-471-70450-8 , pp. 1442-1445.