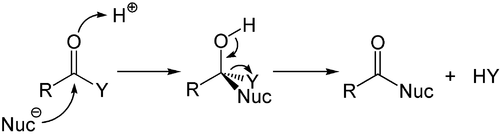
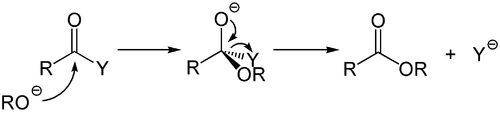
Addition-Elimination Mechanism
The addition-elimination mechanism is a type of reaction in organic chemistry that occurs primarily with derivatives of carboxylic acids . Here, carboxylic acid derivatives such as carboxylic acid halides , carboxylic acid anhydrides , esters , thioesters or amides formally react in a reaction corresponding to the nucleophilic substitution . A nucleophile first adds to the carbonyl group, creating a tetrahedral intermediate product which, with the elimination of a leaving group, reverses the carbonyl function.
The success of the reaction depends on the nucleophilicity of the nucleophile, the electrophilicity of the carboxylic acid derivative and the quality of the leaving group.
mechanism
The overall reaction consists of two sub-steps, addition and elimination, which is why the overall reaction is called the addition-elimination mechanism. An analogous term is nucleophilic substitution on carboxylic acid derivatives .
Acid catalyzed reaction
The reaction can be catalyzed by acids . Both Brønsted and Lewis acids can be used here. The acid interacts with the oxygen atom of the carbonyl function and thus generates a positive partial charge on the carbon atom of the carbonyl, which increases its electrophilicity.
For example, ester hydrolyses or deamidations can be carried out with acid catalysis. The latter plays a role in antibiotic resistance: resistant bacteria have the enzyme penicillinase , which penicillin (a cyclic carboxamide ( lactam )) is able to hydrolyze .
Base-catalyzed reaction
This mechanism is the analogue of acid-induced hydrolysis and is also of great preparative and synthetic importance. These include the base-induced hydrolyses of many carboxylic acid derivatives.
Weak nucleophiles, for example water or alcohols , only react with very electron-poor carboxylic acid derivatives without activation at room temperature. Through the catalytic use of a base , hydroxide ions or alcoholates are formed from the weak nucleophiles , which are significantly better nucleophiles.
The hydrolysis of carboxamides usually takes place poorly, since the amide ion is a poor leaving group. Using hydroxide ions as the nucleophile, a carboxylic acid is formed, which is then deprotonated by the escaped amide ion to form ammonia .
The fact that carboxamides are difficult to hydrolyze is important in biochemistry , since amides are building blocks of peptides and proteins . The stability of the peptide bond , an amide-like bond, is central to all protein biochemistry and enzyme biochemistry.
Hydrolysis under neutral conditions
Carboxylic acid chlorides are among the most reactive and unstable carboxylic acid derivatives. The reason for this is the high electronegativity of chlorine , which polarizes the carbonyl carbon atom so positively that it can easily be attacked nucleophilically. Carboxylic acid chlorides usually react violently with water in an exothermic reaction at room temperature under neutral conditions, whereby hydrochloric acid is formed.
If the compound used is acid-labile, the acid formed can be trapped by using non-nucleophilic bases, for example pyrimidine .
Reactivity
With the same nucleophile, the reactivity of the carboxylic acid derivative depends on the basicity of the leaving group. The electron-rich carboxylates are practically inert .
literature
- Marye Anne Fox / James K. Whitesell: Organic chemistry, fundamentals, mechanisms, bio-organic applications . S. 476 ff., Spektrum, Akad. Verl., 1995, ISBN 3-86025249-6