Carboxylic acids
| Carboxylic acids |
|---|
|
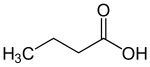
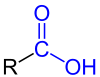
| General structure of the monocarboxylic acids with the carboxy function marked in blue . The radical R represents either a hydrogen atom or an organyl group. |
Carboxylic acids , also called carboxylic acids , are organic compounds that carry one or more carboxy groups (-COOH) and thus have a more or less pronounced acidic character. The salts of carboxylic acids are called carboxylates . Compounds in which the OH group of the carboxy group is replaced by another group, e.g. B. -OR, -NH 2 or -Cl is replaced, carboxylic acid derivatives are called. The three examples mentioned also belong to the carboxylic acid derivatives : carboxylic acid esters , carboxylic acid amides and carboxylic acid halides .
nomenclature
For the systematic naming of the carboxylic acids in German, the word component "acid" is added to the name of the basic structure. Many carboxylic acids also have unsystematic names (common names ), which also end with "acid". Examples of designations of carboxylic acids with the basic structure of an alkane are methanoic acid (formic acid), ethanoic acid (acetic acid) and butanoic acid (butyric acid). The common name denotes the respective carboxylic acid mostly according to a typical occurrence.
Classification
A distinction is made between aliphatic , aromatic and heterocyclic carboxylic acids according to the chemical structure of the radical R to which the group -COOH is bound . The aliphatic carboxylic acids can be further subdivided into alkanoic acids , alkenoic acids and alkynoic acids. Alkanoic acids are also called saturated carboxylic acids . Alkenoic acids, that is to say carboxylic acids with at least one double bond in the remainder (e.g. acrylic acid ) and alkynoic acids with at least one triple bond in the remainder, are, on the other hand, referred to as unsaturated carboxylic acids .
Furthermore, carboxylic acids can be differentiated according to the number of carboxy groups they contain. Monocarboxylic acids have one carboxy group, while dicarboxylic acids (e.g. oxalic acid ) contain two and tricarboxylic acids (e.g. citric acid ) contain three carboxy groups.
There are also groups of carboxylic acids that carry other functional groups in addition to the carboxy group, such as the keto carboxylic acids , the hydroxycarboxylic acids (e.g. lactic acid ) and the amino acids (actually: aminocarboxylic acids ).
From the point of view of chemical structure, the so-called fatty acids are not a special group of carboxylic acids, because they are mostly unbranched, aliphatic, saturated or unsaturated monocarboxylic acids, often with 12 to 22, sometimes, as in butter, even with only 4 Carbon atoms. There they are esterified with glycerine as so-called triglycerides in animal and vegetable fats (see e.g. milk fat ). More recent findings have shown that the fatty lipids of cell membranes also contain shorter-chain and branched carboxylic acids, so that today all carboxylic acids with (chain-like) organyl groups can be summarized under the term fatty acid.
Another group of carboxylic acids identified by their occurrence are the resin acids , which occur in natural resins .
From the point of view of the chemical structure, the so-called metal carboxylic acids do not belong to the group of carboxylic acids. This is the name given to metal complexes with carboxy ligands that occur as intermediate products in ( catalyzed ) reactions with carbon monoxide (CO) and carbon dioxide (CO 2 ), for example in the water-gas shift reaction .
Examples
| aliphatic, saturated monocarboxylic acids |
acetic acid |
Butyric acid (a fatty acid ) |
||
| aliphatic, unsaturated monocarboxylic acids |
Acrylic acid |
Oleic acid (a fatty acid ) |
||
| aliphatic, saturated dicarboxylic acids |
Oxalic acid | Succinic acid | ||
| aliphatic, saturated tricarboxylic acids |
Citric acid | Agaricic acid | ||
| aliphatic, unsaturated dicarboxylic acids |
Fumaric acid | Maleic acid | ||
| aromatic carboxylic acids |
Benzoic acid | Salicylic acid | ||
| heterocyclic carboxylic acids |
Nicotinic acid |
Pyrrolidine-2-carboxylic acid (an amino acid ) |
||
| aliphatic, unsaturated, cyclic monocarboxylic acids |
Abietic acid (a resin acid ) |
Gibberellic acid |
properties
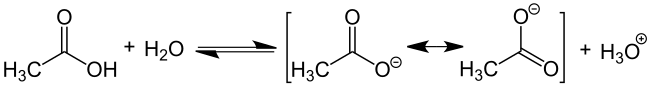
The chemical properties of carboxylic acids without additional functional groups in the alkyl chain are determined solely by the carboxy group. The oxygen atom of the carbonyl group (C = O) has a relatively strong electron-withdrawing effect, so that the bond between hydrogen and the oxygen atom of the hydroxyl group is strongly polarized. This promotes the release of H + ions from the carboxy group, shown here using acetic acid:
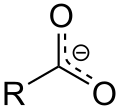
In addition, the acidic character of the carboxylic acids is a consequence of the mesomerism stabilization of the carboxylate anion. It favors the deprotonated form of the carboxylic acid and thus the stability of the anion. Similar to the mesomeric stabilization of benzene , the delocalized electron is often indicated with the following notation (in the example, R stands for the methyl group CH 3 ).
The physical properties of carboxylic acids (acid strength, boiling point or melting point, solubility in water) are largely determined by the type of alkyl chain and any substituents in the alkyl group.
The acidity of a carboxylic acid is more pronounced the shorter the alkyl chain. An additional substituent on the alpha carbon atom with an electron-withdrawing inductive effect (−I effect) increases the acid strength considerably. A more positive partial charge is added to the carboxy group , which can better balance the negative charge of the anion and thus stabilize the anion. An example of this is the more stable trichloroacetate compared to acetate . One measure of the acid strength of a carboxylic acid is the acid dissociation constant or the pK s value
Short-chain carboxylic acids with up to six carbon atoms are colorless, pungent ( formic acid ) or unpleasant ( butyric acid ) smelling liquids with relatively high boiling points. Due to the carboxy group, carboxylic acids have a polar character, which results in good solubility in water, which decreases with increasing length of the alkyl chain. The well possible spatial arrangement of two carboxyl groups favors the formation of intermolecular hydrogen bonds and thus leads to the carboxylic acid dimer . The double mass of the particles in the vapor space above the liquid can explain the relatively high boiling points of carboxylic acids.
With increasing chain length, the lipophilic character of carboxylic acids increases and the hydrophilic character decreases. This also applies to the salts of carboxylic acids. In addition to the lipophilic alkyl chain, with possibly more than six carbon atoms, the salts also have a hydrophilic group in the same molecule with the carboxylate group. The sodium and potassium salts of the long-chain carboxylic acids are therefore amphiphilic substances. They have the properties of surfactants and are used as curd soaps and soft soaps .
Manufacturing
Oxidations
Carboxylic acids can only be produced from alkanes or from aromatic hydrocarbons substituted with alkyl groups by oxidation reactions with strong oxidizing agents if there are no other oxidizable functional groups in the molecule that can also be oxidized, such as e.g. B. hydroxyl or amino groups or CC double bonds, intermediate stages of the complete oxidation of alkyl groups are alcohols and aldehydes , which can therefore also be used as starting substances for oxidation reactions. Known suitable oxidizing agents are oxygen or even ozone , potassium permanganate , chromium trioxide , nitric acid or potassium dichromate .
- Oxidation of alkanes: A process used industrially in Germany from 1930 was the so-called paraffin oxidation . Mixtures of longer-chain alkanes with atmospheric oxygen and permanganates were used at 120 ° C. treated. Oxidative cleavage of the alkane chains resulted in mixtures of different carboxylic acids that had to be separated. Alcohols and aldehydes were produced as by-products. The following associated reaction equation and all following reaction equations for oxidation reactions are simplified schematically.
- Oxidation of alkylated aromatics :
- Oxidation of olefins with basic potassium permanganate. Under neutral conditions, the reaction only leads to vicinal diols .
Reactions with elongation of the alkyl chain
- Reaction of Grignard compounds , previously formed from haloalkanes , with carbon dioxide . The subsequent addition of water leads to the free carboxylic acid. (Reaction equation summarizes both steps schematically).
- If there are functional groups in the alkyl chain that prevent the production of a Grignard compound, a detour is required
- can be selected via the Kolbe nitrile synthesis and subsequent hydrolysis of the nitrile. (Reaction equation shows both steps summarized only schematically)
Hydrolysis of carboxylic acid derivatives
- The hydrolysis of so-called reactive carboxylic acid derivatives, e.g. B. carboxylic acid chlorides or carboxylic acid anhydrides makes no sense, since the reactive carboxylic acid derivatives must always be made from carboxylic acids in order to be able to produce other carboxylic acid derivatives from them . This hydrolysis reaction is therefore a mostly undesirable side reaction that must be avoided when using reactive carboxylic acid derivatives.
- ,
- Alkaline hydrolysis (" saponification ") or acid-catalyzed hydrolysis of carboxylic acid esters :
- The acidic or basic hydrolysis of carboxamides has to take place under severe conditions because of the stability of the carboxamides.
- The acidic or basic hydrolysis of nitriles has to take place under energetic conditions because of the stability of the nitriles.
Other special reactions
- Kolbe-Schmitt reaction : for the synthesis of hydroxybenzoic acids (e.g. salicylic acid ) from phenates and carbon dioxide
- Koch reaction ( hydroformylation ): branched alkenes become highly branched carboxylic acids through the addition of carbon monoxide to the α-C atom
Spectroscopy of carboxylic acids
The most important analytical methods for determining the structure of carboxylic acids are IR and NMR spectroscopy .
In the IR spectrum , the C = O stretching vibration at around 1710–1760 cm −1 and the broad OH stretching vibration around 3000 cm −1 are characteristic.
In the 1 H-NMR spectrum , the acidic hydroxyl protons are shifted to an unusually low field, 10–13 ppm. The protons of the alkyl groups on the carbonyl-C have a chemical shift in the range of approx. 2.0-2.5 ppm; the formic acid hydrogen atom bonded directly to the carbonyl group appears at 8.08 ppm. In a carbon chain of a non-conjugated carboxylic acid, the peaks that are further away from the carbonyl function are increasingly shifted less strongly to the deep field because the influence of the inductive effect of the carbonyl group decreases.
The 13 C-NMR spectrum shows the carboxy carbon atom in the range from about 170 to 180 ppm.
Important reactions
Because of the two oxygen atoms adjacent to the carbon atom of the carboxyl group, which have an electron-withdrawing effect, nucleophilic attacks on the carbon atom of the carboxyl group can take place. The nucleophilic attack can be promoted by acid catalysis (protonation of the carbonyl group) if the attacking nucleophiles are not strong Brønsted bases , e.g. B. Alcohols. When the nucleophiles are bases, e.g. B. ammonia or amines they are protonated and weakened by acid catalysis. Even without acid catalysis, such nucleophiles are protonated by the carboxylic acid itself and become ineffective. At the same time, the corresponding salts of the carboxylic acid are also formed, which can no longer be attacked by nucleophiles.
Esterification
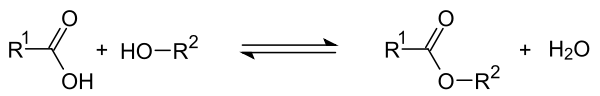
In acid-catalyzed esterification , the carboxyl group of an organic acid reacts with the hydroxyl group of an alcohol. An ester is formed when water is split off . In the course of the reaction, a reverse reaction sets in, because the carboxyl group in the ester formed can also be attacked nucleophilically by the water formed. After the alcohol has been split off, the original acid is then formed back. If R 1 is the remainder of the acid and R 2 is the remainder of the alcohol, the following equilibrium reaction occurs in the course of the reaction :
The position of equilibrium can be influenced. If the ester formed has a lower boiling point than the acid, it can be obtained by continuous distillation. The addition of solid drying agents that can bind water allows the ester formation to proceed more completely.
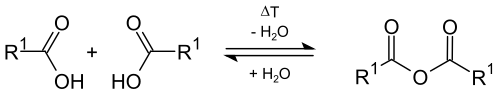
Dehydration
In theory, carboxylic acids can easily be converted into organic carboxylic acid anhydrides with intermolecular elimination of water ( dehydration ) at higher temperatures . In laboratory practice, this reaction is rarely successful, because intramolecular dehydration can also occur at high temperatures, with ketenes being formed.
An alternative to the formation of carboxylic acid anhydrides from carboxylic acids is the addition of strongly dehydrating substances ( phosphorus pentoxide ) or other reactions, such as the reaction of carboxylic acid halides with the salts of carboxylic acids, or the reaction of ketenes with carboxylic acids.
Carboxylic acid anhydrides can react back to the corresponding carboxylic acids with water.
Web links
Individual evidence
- ↑ carboxylic acid at Duden.de, accessed on 15 January 2016th
- ↑ carboxylic acid at Duden.de, accessed on 15 January 2016th
- ↑ a b Brockhaus ABC chemistry. VEB F. A. Brockhaus Verlag, Leipzig 1965, pp. 645-646.
- ↑ G. Wietzel: Production of synthetic fatty acids by oxidation of paraffinic hydrocarbons with molecular oxygen. In: Chemical Engineering Science. 3, 1954, pp. 17-IN4, doi : 10.1016 / S0009-2509 (54) 80003-0 .