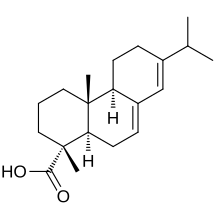
Resin acids
Resin acids or resino acids, formerly also resinole acids, is the name for a highly heterogeneous group of carboxylic acids that form the main components of natural resins . Their salts are called resin soaps or resinates. Depending on the raw material source , they are often based on terpenes , but aromatic carboxylic acids also occur.
They are produced and stored in the plants in special structures and are secondary natural substances . Resin acids have a variety of functions in plants, including protection against pests, mechanical damage and climatic fluctuations.
Occurrence and biosynthesis
In our latitudes, resin acids are mainly found in conifer resins and belong to the group of diterpenes . Pine trees contain numerous radial and vertical channels surrounded by parenchymal tissue that run through the wood and in which the resin collects. In the course of time, the resin collects increasingly in the heartwood and in the sapwood , in the former more in the lower part of the trunk, while the amount of resin in the sapwood increases with the height of the tree. Tritepernharzsäuren may for example be Elemi , dammar and mastic , aromatic acids in Tolubalsam and incense before.
Resin acids are synthesized and stored in various places within the plant and, if necessary, transported to the outside via channels . Their biosynthesis is part of the secondary metabolism of plants, whereby there are two different synthesis pathways. Terpenes are synthesized via the mevalonate pathway in the endoplasmic reticulum and the MEP pathway in the plastids , and aromatic resin acids via the shikimic acid pathway .
properties
Resin acids have alicyclic structures and, in addition to the carboxy group, often also carry other functional groups such as hydroxyl , keto , aldehyde groups or carboxylic acid esters . They are poorly soluble in water but readily soluble in polar organic solvents such as chloroform or diethyl ether . In the resin mixture they are mostly in acid form, but occasionally also as esters. Some of them are very easy to crystallize and melt in the range of 130-200 ° C. With bases (sodium, potassium hydroxide, calcium hydroxide) they form resinates.
Classification
In addition to the resin acids of conifers, which are important for industry and usually have the empirical formula C 20 H 30 O 2 (diterpene acids), triterpene acids and benzoic and cinnamic acid derivatives also occur. What they have in common is their occurrence in natural resins and their origin in the secondary metabolism of plants. In general, resin acids of different botanical origin also come from different chemical groups.
Diterpenes
- Abietans: abietic acid , neoabietic acid , levopimaric acid , palustric acid , dehydroabietic acid
- Pimarans and isopimarans: pimaric acid , sandaracopimaric acid , podocarpic acid
- Labdane: copalic acid, eperuic acid, labdanolic acid, polyalthic acid, pinifolic acid
Triterpenes
- Dammarane: Dammarolic acid
- Tirucallane: (Iso) -Masticadienonic acid, Elemolic acid, Elemonic acid
- Oleanans: oleanonic acid , oleanolic acid , moronic acid, α-boswellic acid
- Ursane: ursolic acid , ursonic acid , β-boswellic acid
- Lupane : lupeolic acid
Others
Various resins contain the aromatic acids cinnamic acid ( acaroid resin ) and benzoic acid ( benzoin , frankincense , tolu balsam , Peru balsam ) as well as the benzyl and other esters of these acids.
use
Sodium and potassium soaps of abietic acid are produced as by-products of the Kraft process through the reaction of wood containing resinous acid with sodium hydroxide. It is a yellow gelatinous paste. If this is mixed with concentrated sulfuric acid , crude oil is produced . This is processed further to obtain the resin and fatty acids it contains. Among other things, tall resin, a substance similar to colophony, is made from the resin acids . It is used as an emulsifier in the manufacture of synthetic rubber , as an additive to adhesives , for printing inks , paints and in construction chemistry . Calcium and zinc resinates are used in paints, lead and manganese resinates as siccatives .
literature
- ^ JH Langenheim: Plant Resins: Chemistry, Evolution, Ecology, and Ethnobotany. Timber Press, Portland and Cambridge 2003, ISBN 978-0-88192-574-6 .
- ↑ Eberhard Breitmaier: Terpenes - aromas, fragrances, pharmaceuticals, pheromones. Wiley-VCH, Weinheim 2005, ISBN 978-3-527-31498-0 .
- ↑ a b Entry on resin soaps. In: Römpp Online . Georg Thieme Verlag, accessed on May 20, 2016.