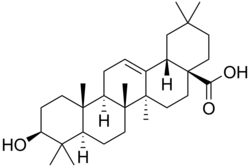
Oleanolic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Oleanolic acid | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 30 H 48 O 3 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 456.71 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
306-308 ° C |
||||||||||||||||||
| solubility |
almost insoluble in water, readily soluble in methanol |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Oleanolic acid is a pentacyclic triterpene made up of five cyclohexane rings . The sapogenin oleanolic acid is a natural component of a number of plants.
Occurrence
Oleanolic acid is a natural substance found in a large number of plants. For example, they can consist of sage ( Salvia officinalis ), rosemary ( Salvia Rosmarinus ), vulgar ivy ( Hedera helix ), sugar beet ( Beta vulgaris ), ginseng ( Panax ginseng ), plantain ( Plantago major ), Syzygium cumini , pistachios ( Pistazia vera ) Olive leaves ( Olea europaea ) and white berry mistletoe ( Viscum album ) are obtained.
Pharmacological properties
Oleanolic acid is weakly cytotoxic and has only a low anti-oxidative potential. Since the pharmacologically interesting properties are relatively weak, various derivatives of oleanolic acid were produced which have a considerably higher potency. A derivative with a significantly higher potential is bardoxolone (2-cyano-3,12-dioxo-l, 9-dien-28-oleanolic acid, CDDO) or its methyl ester (bardoxolon methyl). Bardoxolon is a promising chemopreventive and cytostatic substance that has an anti-proliferative and pro-apoptotic effect. The compound is in clinical testing (phase I / II). Bardoxolon-Methyl is in clinical phase III for the treatment of chronic kidney failure .
Oleanolic acid has been used as an oral therapeutic agent for liver disease in China and Japan since 1977 . Since 1986 against hyperlipidemia and non-lymphatic leukemia . In Japan, an oleanol-containing cream to protect against skin cancer has been on the market since 1990 .
See also
further reading
- A. Bishayee, S. Ahmed, N. Brankov, M. Perloff: Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. In: Frontiers in bioscience Volume 16, 2011, pp. 980-996, PMID 21196213 . PMC 3057757 (free full text). (Review).
- MB Sporn, KT Liby, MM Yore, L. Fu, JM Lopchuk, GW Gribble: New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. In: Journal of Natural Products Volume 74, Number 3, March 2011, pp. 537-545, doi : 10.1021 / np100826q . PMID 21309592 . PMC 3064114 (free full text). (Review).
- JL Ríos: Effects of triterpenes on the immune system. In: Journal of ethnopharmacology Volume 128, number 1, March 2010, pp. 1-14, doi : 10.1016 / j.jep.2009.12.045 . PMID 20079412 . (Review).
- I. Sogno, N. Vannini, G. Lorusso, R. Cammarota, DM Noonan, L. Generoso, MB Sporn, A. Albini: Anti-angiogenic activity of a novel class of chemopreventive compounds: oleanic acid terpenoids. In: Recent results in cancer research. Advances in cancer research. Progrès dans les recherches sur le cancer Volume 181, 2009, pp. 209-212, PMID 19213570 . (Review).
- A. Petronelli, G. Pannitteri, U. Testa: Triterpenoids as new promising anticancer drugs. In: Anti-Cancer Drugs Volume 20, Number 10, November 2009, pp. 880-892, doi : 10.1097 / CAD.0b013e328330fd90 . PMID 19745720 . (Review).
- MN Laszczyk: Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. In: Planta Medica Volume 75, Number 15, December 2009, pp. 1549-1560, doi : 10.1055 / s-0029-1186102 . PMID 19742422 . (Review).
- B. Yu, J. Sun: Current synthesis of triterpene saponins. In: Chemistry, an Asian journal Volume 4, Number 5, May 2009, pp. 642–654, doi : 10.1002 / asia.200800462 . PMID 19294723 . (Review).
- N. Sultana, A. Ata: Oleanolic acid and related derivatives as medicinally important compounds. In: Journal of Enzyme Inhibition and Medicinal Chemistry Volume 23, Number 6, December 2008, pp. 739-756, doi : 10.1080 / 14756360701633187 . PMID 18618318 .
- J. Liu: Oleanolic acid and ursolic acid: research perspectives. In: Journal of ethnopharmacology Volume 100, number 1-2, August 2005, pp. 92-94, doi : 10.1016 / j.jep.2005.05.024 . PMID 15994040 . (Review).
- Z. Ovesná, A. Vachálková, K. Horváthová, D. Tóthová: Pentacyclic triterpenoic acids: new chemoprotective compounds. Mini review. In: Neoplasma Volume 51, Number 5, 2004, pp. 327-333, PMID 15640935 . (Review).
Individual evidence
- ↑ Entry on OLEANOLIC ACID in the CosIng database of the EU Commission, accessed on May 18, 2020.
- ↑ a b T. Mühle: Separation of the positional isomers oleanolic acid and ursolic acid. Diplomica Verlag, 2009, ISBN 3-836-67870-5 , p. 7 ( limited preview in Google book search).
- ↑ a b c data sheet Oleanolic acid from Sigma-Aldrich , accessed on April 24, 2011 ( PDF ).
- ↑ Dr. PN Ravindran : The Encyclopedia of Herbs and Spices (ingredients, triterpenes in the drug) In: Google Books , 2017, p. 812.
- ↑ Concentrated sage - effectively extracting active ingredients from the ancient medicinal plant ( Page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. (PDF; 851 kB) Münster University of Applied Sciences, pp. 26–27.
- ↑ a b H. Schmandke : Triterpenoids in olives. (PDF; 283 kB) In: Ernahrung Umschau 2, 2009, pp. 92–95.
- ↑ S. Jäger, A. Scheffler, H. Schmellenkamp: Pharmacology of selected terpenes. In: Pharmazeutische Zeitung 22, 2006.
- ↑ A. Bishayee, S. Ahmed, N. Brankov, M. Perloff: Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. In: Frontiers in bioscience Volume 16, 2011, pp. 980-996, PMID 21196213 . PMC 3057757 (free full text). (Review).
- ↑ D. Deeb, X. Gao, Y. Liu, D. Jiang, GW Divine, AS Arbab, SA Dulchavsky, SC Gautam: Synthetic triterpenoid CDDO prevents the progression and metastasis of prostate cancer in TRAMP mice by inhibiting survival signaling. In: Carcinogenesis Volume 32, Number 5, May 2011, pp. 757-764, doi : 10.1093 / carcin / bgr030 . PMID 21325633 . PMC 3086702 (free full text).
- ↑ Clinical study (phase I / II): CDDO in Treating Patients With Metastatic or Unresectable Solid Tumors or Lymphoma at Clinicaltrials.gov of the NIH
- ↑ A. Petronelli, E. Pelosi, S. Santoro, E. Saulle, AM Cerio, G. Mariani, C. Labbaye, U. Testa: CDDO-Im is a stimulator of megakaryocytic differentiation. In: Leukemia Research Volume 35, Number 4, April 2011, pp. 534-544, doi : 10.1016 / j.leukres.2010.09.013 . PMID 21035854 .
- ↑ Clinical study (phase III): Bardoxolone Methyl Evaluation in Patients With Chronic Kidney Disease and Type 2 Diabetes (BEACON) at Clinicaltrials.gov of the NIH
Web links
- Pharmacology of selected terpenes. Pharmaceutical Newspaper

