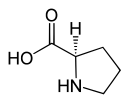
Proline
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| Structural formula of naturally occurring L- proline | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Proline | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 9 NO 2 | |||||||||||||||
| Brief description |
colorless solid with an amine-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 115.13 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.35–1.38 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
Decomposition: 220–222 ° C ( D - and L -form) |
|||||||||||||||
| pK s value |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
L- proline , abbreviated to Pro or P , [systematic name: ( S ) -pyrrolidine-2-carboxylic acid] is a non-essential proteinogenic heterocyclic secondary α - amino acid and is, because of its biosynthesis, from pyrroline-2-carboxylic acid [more precisely: ( S ) - 3,4-dihydro-2 H - pyrrole -2-carboxylic acid] sometimes incorrectly referred to as imino acid (a classification that is now obsolete).
Stereoisomerism
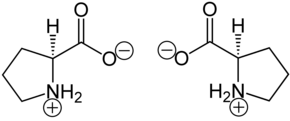
In the proteins, in addition to other amino acids, only L- proline [synonym: ( S ) -proline] is peptide- bound. The enantiomer of this is the mirror-image D- proline [synonym: ( R ) -proline]. Racemic DL- proline [synonyms: ( RS ) -proline or (±) -proline] is of little importance.
Whenever “proline” is mentioned in this text or in the scientific literature without any additional name ( prefix ), L- proline is meant.
| Isomers of proline | ||
| Surname | L -proline | D -proline |
| other names | ( S ) -proline | ( R ) -proline |
| Structural formula |  |
|
| CAS number | 147-85-3 | 344-25-2 |
| 609-36-9 ( DL ) | ||
| EC number | 205-702-2 | 206-452-7 |
| 210-189-3 ( DL ) | ||
| ECHA info card | 100.005.185 | 100.005.866 |
| 100.009.264 ( DL ) | ||
| PubChem | 145742 | 8988 |
| 614 ( DL ) | ||
| DrugBank | DB00172 | - |
| - ( DL ) | ||
| Wikidata | Q20035886 | Q20035962 |
| Q484583 ( DL ) | ||
history
The name proline comes from Emil Fischer and is derived from pyrrolidine .
Occurrence
Within proteins it occurs in both cis and trans -bound configurations . L- proline has a considerable influence on the folding of proteins, since like glycine it can interrupt α-helices and β-sheets due to the frequently occurring cis -peptide bond . It can also form its own motifs, which often act as signal sequences for other proteins.
biosynthesis
For the biosynthesis including structural formulas see section web links
L- proline is produced biochemically from L - glutamate . This requires a total of one molecule of ATP and two molecules of reduction equivalents in the form of NADPH .
L- proline is the precursor of L - hydroxyproline , which is formed with the involvement of vitamin C after incorporation into collagen and determines its mechanical properties (deficiency disease: scurvy ). Catalysed by a hydroxylase , prolyl residues are modified either on the β or on the γ atom of the tetrahydropyrrole ring, depending on their position in the protein .
properties
Proline is mainly present as an “inner salt” or zwitterion , the formation of which can be explained by the fact that the proton of the carboxy group migrates to the lone pair of electrons on the nitrogen atom of the amino group . The decomposition temperature is> 185 ° C.
The zwitterion does not migrate in the electric field because it is uncharged as a whole. Strictly speaking, this is the case at the isoelectric point (a certain pH value), at which the proline also has its lowest solubility in water.
Because the nitrogen atom of the proline in the peptide group is not connected to an H atom, no hydrogen bond can be formed. A disruption of the secondary structure (kink or curvature) occurs at such points in a polypeptide chain.
Other features:
- Side chain : hydrophobic
- Isoelectric point : pH = 6.30
- Van der Waals volume : 90 Å 3
- Lipid solubility : LogP = −1.6
Functions
L- proline is produced in the human body e.g. B. for the formation of collagen , the protein that makes up connective tissue and bones. It is also referred to as a “helix breaker” and is often found at the transition from an alpha helix to another secondary structure (often random coil ). Proline is the only amino acid whose peptide bond does not have a hydrogen atom. As a result, it cannot participate in hydrogen bonding. L- proline is used as a biomarker in ecotoxicology , e.g. B. for drought stress , salt stress , as it is increasingly produced by plants when the water balance comes under stress. L- proline as a cyclic amino acid acts as a buffer against some ions that could otherwise restrict the enzyme activities in the cytoplasm .
Chemical use
Enantiomerically pure proline, as an element of the chiral pool, is the starting substance for the synthesis of the Evans auxiliary , the CBS reagent , the Enders reagent (RAMP / SAMP) and is a widely used catalyst in organocatalysis . The literature on the use of ( S ) - or ( R ) -proline and their derivatives in stereoselective synthesis is extensive. An L- proline derivative is used as a chiral selector in enantioselective thin-layer chromatography . The therapeutically and economically important drug captopril is synthesized from L- proline.
literature
- Hans Beyer and Wolfgang Walter : Textbook of Organic Chemistry , 20th Edition, S. Hirzel Verlag, Stuttgart 1984, ISBN 3-7776-0406-2 .
- Hans-Dieter Jakubke and Hans Jeschkeit: Amino acids, peptides, proteins , Verlag Chemie, Weinheim 1982, ISBN 3-527-25892-2 .
- Jesse Philip Greenstein and Milton Winitz: Chemistry of Amino Acids , John Wiley & Sons, 1962, Volumes 1 to 3, ISBN 0-471-32637-2 .
- Yoshiharu Izumi, Ichiro Chibata and Tamio Itoh: Production and Utilization of Amino Acids , Angewandte Chemie International Edition in English, 17 (1978), pp. 176-183.
- Karlheinz Drauz, Axel Kleemann and Jürgen Martens : Induction of Asymmetry by Amino Acids , Angewandte Chemie 94 (1982), pp. 590-613; Angewandte Chemie International Edition, English 21 (1982), pp. 584-608.
- Jürgen Martens: Asymmetric Syntheses with Amino Acids , Topics in Current Chemistry / Advances in Chemical Research 125 (1984), pp. 165–246.
- Jürgen Martens: Induction of asymmetry by imino acids , Chemiker-Zeitung 110 (1986), pp. 169-183.
- Gary M. Coppola and Herbert F. Schuster: Asymmetric Synthesis - Construction of Chiral Molecules using Amino Acids , Wiley, New York 1987, pp. 267-345.
- Peter I. Dalko: Do We Need Asymmetric Organocatalysis? , Chimia 61 (2007), pp. 213-218.
- Kurt Günther, Jürgen Martens and Maren Schickedanz: Thin-layer chromatographic separation of enantiomers by means of ligand exchange , Angewandte Chemie 96 (1984), pp. 514-515; Angewandte Chemie International Edition English 23 (1984), p. 506.
- Kurt Günther, Maren Schickedanz and Jürgen Martens: Thin-Layer Chromatographic Enantiomeric Resolution , Naturwissenschaften 72 (1985), pp. 149–150.
- Kurt Günther, Jürgen Martens and Maren Schickedanz: Resolution of Optical Isomers by Thin-Layer Chromatography (TLC). Enantiomeric Purity of L-DOPA , Fresenius Zeitschrift für Analytische Chemie 322 (1985), pp. 513-514.
- J. Behre, R. Voigt, I. Althöfer, S. Schuster: On the evolutionary significance of the size and planarity of the proline ring . Natural Sciences 99 (2012) 789–799.
Web links
Individual evidence
- ↑ a b c d e f g h Proline data sheet (PDF) from Merck , accessed on December 23, 2019.
- ^ A b F. A. Carey: Organic Chemistry , 5th edition, The McGraw Companies 2001, p. 1059, Link
- ↑ Nicolai Marroquin: Molecular genetic analysis of the FGF8 gene in patients with diseases from the congenital cranial dysinnervation disorders (CCDDs) . GRIN Verlag, 2011, ISBN 978-3-656-01631-1 . P. 36 ( limited preview in Google Book search).
- ↑ PM Hardy: The Protein Amino Acids in GC Barrett (editor): Chemistry and Biochemistry of the Amino Acids , Chapman and Hall, 1985, ISBN 0-412-23410-6 , p. 9.
- ↑ a b Catherine H. Wu, Jerry Wayne McLarty: Neural Networks and Genome Informatics. Elsevier, 2000, ISBN 978-0-08-042800-0 . P. 70 ( limited preview in Google Book search).
-
↑ Selected review articles:
(a) Dieter Seebach , Albert K. Beck, D. Michael Badine, Michael Limbach, Albert Eschenmoser , Adi M. Treasurywala, Reinhard Hobi, Walter Prikoszovich and Bernhard Linder: Are Oxazolidinones really unproductive, parasitic species in proline catalysis ? Thoughts and experiments pointing to an alternative view , In: Helvetica Chimica Acta 90 (2007), pp. 425-471.
(b) Santanu Mukherjee, Jung Woon Yang, Sebastian Hoffmann and Benjamin List : Asymmetric enamine catalysis , In: Chemical Reviews 107 (2007), pp. 5471-5569.
(c) Peter Diner, Anne Kjaersgaard, Mette Alstrup Lie and Karl Anker Jørgensen: On the origin of the stereoselectivity in organocatalysed reactions with trimethylsilyl-protected diarylprolinol , In: Chemistry - A European Journal 14 , (2008), pp. 122–127 .
(d) Yujiro Hayashi, Hiroaki Gotoh, Ryouhei Masui and Hayato Ishikawa: Diphenylprolinol silyl ether as a catalyst in an enantioselective, catalytic, formal aza [3 + 3] cycloaddition reaction for the formation of enantioenriched piperidines , In: Angewandte Chemie-International Edition 47 (2008), pp. 4012-4015.
(e) Hiyoshizo Kotsuki, Hideaki Ikishima and Atsushi Okuyama: Organocatalytic asymmetric synthesis using proline and related molecules. Part 1 , In: Heterocycles 75 (2008), pp. 493-529.
(f) Antonia Mielgo and Claudio Palomo: α, α-Diarylprolinol ethers: New tools for functionalization of carbonyl compounds , In: Chemistry - An Asian Journal 3 (2008), pp. 922-948. - ↑ Kurt Günther, Peter Richter and Klaus Möller: Separation of Enantiomers by Thin-Layer Chromatography , pages 29-59 in Methods in Molecular Biology ™ - Chiral Separations , 243 (2004), Springer Verlag, ISBN 978-1-58829-150- 9 .