GPCR oligomer
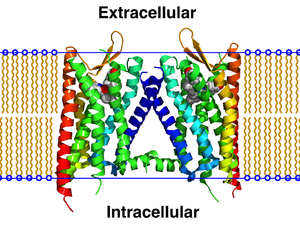
A GPCR oligomer is an association or complex, known as an oligomer , of several G protein-coupled receptors that are in direct contact with one another and held together by atomic bonds or intermolecular forces . Receptors within the association are called protomers , while unconnected receptors are called monomers. Receptor homomers are composed of identical protomers , heteromers of dissimilar protomers. Receptor associations, which as such, but not in the form of their parent monomers, are natively capable of stimulus transmission, are referred to as constitutive receptors . Receptors that are only indirectly connected to one another are not referred to as oligomers. The functional effect of a ligand bond that is transferred from one protomer to one or more other protomers is called crosstalk . The specific type of functional interaction of ligands, which results from binding to two or more protomers of a complex, is expressed as cooperativity .
The existence of receptor oligomers is a general phenomenon that has superseded the long prevailing paradigmatic notion of the function of receptors as pure monomers and the discovery of which has far-reaching consequences for the understanding of neurobiological diseases and for the development of drugs. Receptor oligomers and their function in the interactome are intensively researched according to their importance.
The oligomerization is not limited to G-protein-coupled receptors, but is also observed on other target proteins, such as. B. plasmalemmal transporters and ion channels . Cross-group functional interaction is possible.
Discovery story
For a long time it was assumed that receptors transmitted effects only in their basic functional forms - as monomers . The first evidence of the existence of GPCR oligomers dates back to 1975. Lefkowitz and co-workers had observed a behavior in beta-adrenoceptors known as negative cooperativity, which is based on the existence of receptor dimers or oligomers. At the beginning of the 1980s it was hypothesized that receptors could form larger associations, so-called mosaics, or that two receptors could interact directly with one another. Mass determinations of beta-adrenoceptors (1982) and muscarinic receptors (1983) indicated that the receptors can exist in homodimeric or homodimeric forms. In 1991 phenomena were observed that can be interpreted as crosstalk and thus indicated receptor heteromer expression. The study focused adenosine A 2A - and dopamine D 2 receptors . In 1993, Maggio and co-workers demonstrated the ability of two G protein-coupled receptors to heteromerize by using chimeras of muscarinic M 3 receptors and α 2C adrenoceptors .
In 2005, evidence was provided that receptor oligomers are of functional importance in the living organism. The crystal structure of a CXCR4 dimer was published in 2010.
Properties of the oligomers
Effect of oligomerization
GPCR oligomers consist of dimers, trimers, tetramers or higher order associations. The oligomers are to be viewed as entities that have properties that differ more or less and in many ways from those of the monomers. The functional character of a receptor depends on its tertiary or quaternary structure . If receptors touch each other over a larger area or at sensitive points, forces act that change the shape as well as the inner mobility of the protomers now; In short, protomers act as allosteric modulators on one another. This has consequences for:
- supplying the cell surface with receptors
- the ligand binding at various binding sites
- the G protein coupling
- intracellular traffic (compare signal transduction )
- the modification of the desensitization profiles
- the tendency to endocytosis and internalization
- the post-endocytotic fate of the receptors
It is currently unclear whether all receptor oligomers have a functional role in signal transmission.
See also
literature
- R. Rozenfeld, LA Devi: Exploring a role for heteromerization in GPCR signaling specificity. In: Biochem J . Volume 433, 2011, p. 11. PMID 21158738 .
- NJ Smith, G. Milligan: Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. In: Pharmacological reviews. Volume 62, number 4, December 2010, pp. 701-725, doi: 10.1124 / pr.110.002667 . PMID 21079041 , PMC 2993260 (free full text) (review).
- J. González-Maeso: GPCR oligomers in pharmacology and signaling. In: Molecular brain. Volume 4, number 1, 2011, p. 20, doi: 10.1186 / 1756-6606-4-20 . PMID 21619615 , PMC 3128055 (free full text) (review).
- J. Giraldo, JP Pin: G Protein-coupled Receptors: From Structure to Function . Royal Society of Chemistry, 2011, ISBN 978-1-84973-183-6 .
- A. Gilchrist: GPCR Molecular Pharmacology and Drug Targeting: Shifting Paradigms and New Directions . John Wiley & Sons, 2010, ISBN 978-1-118-03517-7 .
References and comments
- ↑ H. Wu et al.: Structure of the human κ-opioid receptor in complex with JDTic. In: Nature . 2012. Epub. PMID 22437504 .
- ↑ In quasi-homomers, individual protomers are modified, but the modification has no functional effect.
- ↑ Smith and Milligan (2010) describe cooperativity as the effects of multiple equivalents of the same ligand that binds to multiple, generally identical, binding sites.
- ↑ L. Albizu, JL Moreno, J. González-Maeso, SC Sealfon: Heteromerization of G protein-coupled receptors: relevance to neurological disorders and neurotherapeutics. In: CNS Neurol Disord - Drug Targets . 2010, p. 636. PMID 20632964 .
- ^ R. Rozenfeld, LA Devi: Receptor heteromerization and drug discovery. In: Trends Pharmacol Sci . 2010, p. 124. PMID 20060175 , PMC 2834828 (free full text)
- ↑ JA Schmid et al .: Oligomerization of the human serotonin transporter and of the rat GABA transporter 1 visualized by fluorescence resonance energy transfer microscopy in living cells. In: J Biol Chem . 2001, p. 3805. PMID 11071889 .
- ↑ T. Sorkina, S. Doolen, E. Galperin, NR Zahniser, A. Sorkin: Oligomerization of dopamine transporters visualized in living cells by fluorescence resonance energy transfer microscopy. In: J Biol Chem. 2003, p. 28274. PMID 12746456 .
- ↑ M. Wang et al .: Schizophrenia, amphetamine-induced sensitized state and acute amphetamine exposure all show a common alteration: increased dopamine D2 receptor dimerization. In: Mol Brain. 2010, p. 25, section "Discussion". PMID 20813060 .
- ↑ LE Limbird, PD Meyts, RJ Lefkowitz: beta-adrenergic receptors: evidence for negative cooperativity. In: Biochem Biophys Res Commun . 1975, p. 1160. PMID 1137592 .
- ↑ K. Fuxe et al .: GPCR heteromers and their allosteric receptor-receptor interactions. In: Curr Med Chem . 2012, p. 356. PMID 22335512 .
- ^ NJ Birdsall: Can different receptors interact directly with each other? In: Trends Neurosci. 1982, p. 137. ScienceDirect .
- ↑ CM Fraser, JC Venter: The size of the mammalian lung beta 2-adrenergic receptor as determined by target size analysis and immunoaffinity chromatography. In: Biochem Biophys Res Commun. 1982, p. 21. PMID 6297476 .
- ↑ S. Avissar, G. Amitai, M. Sokolovsky: Oligomeric structure of muscarinic receptors is shown by photoaffinity labeling: subunit assembly may explain high- and low-affinity agonist states. In: PNAS . 1983, p. 156. PMID 6571990 .
- ^ S. Ferre, G. von Euler, B. Johansson, BB Fredholm, K. Fuxe: Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. In: PNAS. 1991, p. 7238. PMID 1678519 , PMC 52269 (free full text).
- ^ R. Maggio, Z. Vogel, J. Wess: Coexpression studies with mutant muscarinic / adrenergic receptors provide evidence for intermolecular "cross-talk" between G-protein-linked receptors. In: PNAS. 1993, p. 3103. PMID 8385357 , PMC 46245 (free full text).
- ↑ M. Waldhoer et al: A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. In: PNAS. 2005, p. 9050. PMID 15932946 , PMC 1157030 (free full text).
- ↑ B. Wu et al .: Structures of the CXCR4 Chemokine GPCR with Small-Molecule and Cyclic Peptide Antagonists. In: Science . 2010, p. 1066. PMID 20929726 .
