Ibogaine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
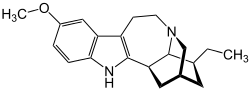
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Ibogaine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 20 H 26 N 2 O | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 310.44 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
153 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Ibogaine is an indole alkaloid with hallucinogenic effects in the broader sense . It occurs in various dog venom plants , especially in Tabernanthe iboga .
history
Ibogaine was first extracted from the root bark of Tabernanthe iboga in 1901 by Dybowski and Landrin as well as by Haller and Heckel .
In the same year, French pharmacologists observed an unusual type of arousal in animals. Phisalix suspected hallucinogenic effects due to the changed behavior of dogs. After further clinical tests, the alkaloid was recommended to support convalescence and neurasthenia , but was then rarely used. In the 1940s, Raymond-Hamet and colleagues published studies of the pharmacological effects on isolated cell tissue and the cardiovascular system.
In France, a stimulant called Lambarene was sold from 1939 to 1967 . One tablet contained 8 mg of ibogaine, which was extracted from Tabernanthe manii , a relative of Tabernanthe iboga . Another ibogaine extract, Iperton, was sold as a tonic .
The demanding total synthesis was accomplished by George Büchi in 1966 . Since then, other totally synthetic approaches have been developed, all of which are only of academic interest.
Iboga and ibogaine have been banned in the USA since 1967 (Schedule I). In 1989 ibogaine was added to the doping list of the International Olympic Committee .
effect
Ibogaine has a stimulating effect in small doses. Higher doses (from 5–10 mg / kg body weight) trigger visions, i. In other words, when the eyes are closed, rapid sequences of images and films are seen in a kind of dream-like experience, often with intense emotional and religious-mystical feelings. Hallucinations with open eyes, however, rarely occur. Therefore, the effect is not comparable to that of better known psychedelics such as LSD . It was suggested that instead of “hallucinogenic” the word “oneirogen” , i.e. H. dreamy to use. The effect lasts between eight and twelve hours, with the acute visionary phase only lasting four to eight hours. Around a fifth of consumers report subjective after-effects 24 hours after ingestion, 15 percent even after 36 hours. Even higher doses lead to cramps, symptoms of paralysis and can lead to death from respiratory failure. Furthermore, there is the risk of cardiac arrhythmias , which in the worst case can lead to sudden cardiac death .
Ibogaine lowers blood pressure, appetite and digestive activity and is a weak acetylcholinesterase inhibitor .
There are people who are allergic to ibogaine; therefore, a small test dose should always be used initially in order to await a possible reaction.
Medical use
Use for drug withdrawal
In the 1960s, Howard Lotsof discovered the addiction-disrupting or addiction-reducing effects of ibogaine and received several US patents for therapy with ibogaine in the 1980s and 1990s. One researched effect is the improvement of withdrawal symptoms in opiate withdrawal as well as the potential benefit in the treatment of nicotine, methamphetamine, alcohol and other substance addiction.
Since the mid-1980s, self-help organizations and private individuals, but also doctors, have been offering withdrawal with ibogaine, both in clinical and informal settings. While ibogaine is not approved as a drug in most countries, it is not illegal either, a black market has formed in the USA due to the ban there. Nevertheless, it was used as an aid in psychotherapy.
The exact mechanism by which the alkaloid is supposed to break dependencies is not known. Test subjects who were administered ibogaine repeatedly described that they had relived situations during the intoxication which they believed were decisive for their dependence. Others shared visions that helped them identify and overcome the fears underlying their addiction.
From 1985 onwards, several patents were granted on ibogaine for chemical withdrawal, as well as on cocaine and amphetamine, alcohol, nicotine, and multiple addictions.
Pain management
In 1957, Jurg Schneider, pharmacologist at CIBA, described that ibogaine potentiated the analgesic effect of morphine. No further research was done until almost 50 years later Patrick Kroupa and Hattie Wells published the first protocol of concomitant administration of ibogaine with opiates to humans, which suggests that ibogaine reduces tolerance. It should be noted that potentiating the effects of ibogaine can be a very risky procedure.
psychotherapy
Ibogaine was introduced as an accompaniment for psychotherapy by Claudio Naranjo and published in his book The Healing Journey . In 1974 he was granted the patent CA939266.
pharmacology
Ibogaine is said to enable a relatively quick and painless withdrawal from opiates . More recent studies indicate an increase in the nerve growth factor GDNF (Glial Cell Line-Derived Neurotrophic Factor) in the brain. In animal experiments it could be shown that rats accustomed to alcohol with increased GDNF levels in the brain consumed less ethanol and had a lower relapse rate than an untreated control group even after a two-week period of abstinence.
Ibogaine is metabolized in the liver to noribogain (12-hydroxyibogamine), which is subject to a moderate depot effect. Today it is not assumed that noribogain plays the central role in the abstinence phenomenon.
Due to side effects and toxicological concerns, the use of ibogaine as a medicinal product is unlikely in the future either. Efforts to develop improved active ingredients and also to explain the mechanism of action of the abstinence phenomenon led to a number of synthetic ibogaine derivatives , the derivative 18-methoxycoronaridine showing significantly fewer side effects than ibogaine in animal experiments. Pharmacologically, these compounds have in common that they inhibit the nicotine receptor of the α3β4 type .
The inhibitory effect on the hERG channel leads to QT prolongation in the heart .
Ibogaine inhibits the serotonin transporter in a non-competitive and non- covalent manner, and thus differs from all previously known inhibitors of plasmalemmal monoamine transporters.
Legal position
In the United States, ibogaine is a Class I substance regulated under the Controlled Substances Act .
Ibogaine is also regulated as a class I substance in Sweden.
In Norway, ibogaine falls under Appendix 10 of the local regulations on handling narcotics and is therefore illegal.
Web links
- Ibogaine in Addiction Therapy , lecture by Sandra Karpetas (Iboga Therapy House, Canada), given at the Entheovision Congress 2004
Individual evidence
- ↑ Chemical Structure and Properties on ibogainedossier.com, accessed on March 23, 2015 (English).
- ↑ a b Datasheet Ibogaine hydrochloride from Sigma-Aldrich , accessed on April 4, 2011 ( PDF ).
- ↑ Entry on Ibogaine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ J. Dybowski, E. Landrin CR Acad. Sci. 1901 , Vol. 133, p. 748 ( Memento of July 25, 2006 in the Internet Archive ). (pdf; 61 kB).
- ↑ G. Büchi: The Total Synthesis of Iboga Alkaloids. In: J. Am. Chem. Soc. 1966, Vol. 88 (13), pp. 3099-3109. (pdf, English; 2.1 MB) .
- ↑ C. Frauenfelder: New access to the iboga alkaloids . Zurich 1999, p. 24 , doi : 10.3929 / ethz-a-003839200 (dissertation, ETH Zurich).
- ↑ a b Koenig X, Kovar M, Boehm S, Sandtner W, Hilber K: Anti-addiction drug ibogaine inhibits hERG channels: a cardiac arrhythmia risk . In: Addict Biol . 2012. doi : 10.1111 / j.1369-1600.2012.00447.x . PMID 22458604 .
- ^ Paling FP, Andrews LM, Valk GD, Blom HJ: Life-threatening complications of ibogaine: three case reports . In: Neth J Med . 70, No. 9, 2012, pp. 422-4. PMID 23123541 .
- ↑ Alper K, Reith ME, Sershen H: Ibogaine and the inhibition of acetylcholinesterase . In: J Ethnopharmacol . 139, No. 3, 2012, pp. 879-82. doi : 10.1016 / j.jep.2011.12.006 . PMID 22200647 .
- ↑ Dosing. In: ibogainealliance.org. Global Ibogain Therapy Alliance, accessed February 21, 2017 .
- ↑ KR Alper, HS Lotsof, GM Frenken, DJ Luciano, J. Bastiaans 1999 234–42.
- ^ Giannini, A. James: Drugs of Abuse , 2nd. Edition, Practice Management Information Corporation, 1997, ISBN 1-57066-053-0 .
- ↑ KR Alper, HS Lotsof, GM Frenken, DJ Luciano, J. Bastiaans 1999 234–42.
- ↑ HS Lotsof (1995). Ibogaine in the Treatment of Chemical Dependence Disorders: Clinical Perspectives (Originally published in MAPS Bulletin (1995) V (3): 19-26).
- ↑ a b Naranjo, Claudio: V, Ibogaine: Fantasy and Reality . In: The healing journey: new approaches to consciousness . Pantheon Books, New York 1973, ISBN 0-394-48826-1 , pp. 197-231. Archived from the original on December 4, 2008 (Retrieved October 10, 2010).
- ^ Rapid method for interrupting the narcotic addiction syndrome - US 4,499,096 (1985) .
- ↑ Rapid method for interrupting the cocaine and amphetamine abuse syndrome "- US 4,587,243 (1986) .
- ↑ Rapid method for attenuating the alcohol dependency syndrome "US 4,957,523 (1989) .
- ↑ Rapid method for interrupting or attenuating the nicotine / tobacco dependency syndrome - US 5,026,697 (1991) .
- ↑ Rapid method for interrupting or attenuating poly-drug dependency syndromes, US 5,124,994 (1992) .
- ↑ Tabernanthine, Ibogaine Containing Analgesic Compositions - US 2817623 ( Memento of March 19, 2012 in the Internet Archive ).
- ↑ Kroupa, Patrick K. and Wells, Hattie: Ibogaine in the 21st Century (PDF) Multidisciplinary Association for Psychedelic Studies. Pp. 21-25. 2005. Retrieved January 23, 2015.
- ↑ MEDICAMENT AGISSANT AU NIVEAU DU SYSTEME NERVEUX CENTRAL UTILISABLE DANS LES TRAITEMENTS PSYCHOTERAPIQUES ET COMME ANTI-DROGUE, CA939266 .
- ↑ ibeginagain.org: "Ibogain Therapy Questions and Answers" ( Memento from January 16, 2012 in the Internet Archive )
- ↑ Dao-Yao He, Nancy NH McGough, Ajay Ravindranathan, Jerome Jeanblanc, Marian L. Logrip, Khanhky Phamluong, Patricia H. Janak, Dorit Ron: Glial Cell Line-Derived Neurotrophic Factor Mediates the Desirable Actions of the Anti-Addiction Drug Ibogaine against Alcohol Consumption . In: Journal of Neuroscience . tape 25 , no. 3 , 2005, p. 619-628 , doi : 10.1523 / JNEUROSCI.3959-04.2005 , PMID 15659598 .
- ↑ SD Glick: Ibogaine-like effects of noribogaine in rats . In: Brain Res. . 713 (1-2), 1996, pp. 294-7. PMID 8725004 .
- ↑ Arias HR, Rosenberg A, Targowska-Duda KM, et al. : Interaction of ibogaine with human alpha3beta4-nicotinic acetylcholine receptors in different conformational states . In: Int. J. Biochem. Cell Biol . 42, No. 9, 2010, pp. 1525-35. PMID 20684041 .
- ↑ OD Tarashenko: Is antagonism of alpha3beta4 nicotinic receptors a strategy to reduce morphine dependence? . In: Eur. J. Pharmacol. . 513 (3), 2005, pp. 207-18. PMID 15862802 .
- ^ CJ Pace: Novel iboga alkaloid congeners block nicotinic receptors and reduce drug self-administration . In: Eur. J. Pharmacol. . 492 (2-3), 2004, pp. 159-67. PMID 15178360 .
- ↑ IM Maisonneuve (2003). Anti-addictive actions of an iboga alkaloid congener: a novel mechanism for a novel treatment. Pharmacol. Biochem. Behav. 75 (3) , 607-18 (Eng.).
- ↑ Hoelen DW, Spiering W, Valk GD: Long-QT syndrome induced by the antiaddiction drug ibogaine . In: N. Engl. J. Med. . 360, No. 3, 2009, pp. 308-9. doi : 10.1056 / NEJMc0804248 . PMID 19144953 .
- ↑ Bulling S, Schicker K, Zhang YW, et al. : The mechanistic basis for noncompetitive ibogaine inhibition of serotonin and dopamine transporters . In: J. Biol. Chem. . 287, No. 22, 2012, pp. 18524-34. doi : 10.1074 / jbc.M112.343681 . PMID 22451652 . PMC 3365767 (free full text).
- ↑ Scheduling Actions Controlled Substances Regulated Chemicals .
- ↑ Läkemedelsverkets föreskrifter om förteckningar över narcotics; 1997: 12 ( memento from September 16, 2018 in the Internet Archive ).
- ↑ Forskrift om narcotics (narcotics writings) . In: lovdata.no . Retrieved March 24, 2015.

