Loperamide
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
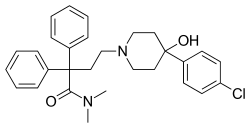
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Loperamide | |||||||||||||||||||||
| other names |
4- [4- (4-chlorophenyl) -4-hydroxypiperidin-1-yl] - N , N -dimethyl-2,2-diphenylbutyramide ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 29 H 33 ClN 2 O 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 477.04 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
222–223 ° C (monohydrochloride) |
|||||||||||||||||||||
| pK s value |
8.6 |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Loperamide is a drug from the group of peristalsis inhibitors that is used for the symptomatic treatment of diarrheal diseases of various causes in adolescents from 12 years of age and adults. Loperamide (2 mg tablets) has been on the World Health Organization's list of essential drugs since 2013 (currently: WHO Model List of Essential Medicines 19th List 2015 ).
history
Loperamide was first synthesized in 1969 by Paul Janssen ( Janssen Pharmaceutica ) in Beerse, Belgium . The first clinical study with loperamide was published in 1973 in the Journal of Medicinal Chemistry. In 1973 loperamide was brought onto the market under the trade name Imodium . Loperamide has been available in Germany since 1976.
scope of application
Loperamide (loperamide hydrochloride) is the best-selling non-prescription antidiarrheal on the German market. For diarrhea ( diarrhea ), the symptomatic treatment is paramount. Typical therapeutic measures are: arranging sufficient oral fluid / electrolyte intake, medical consultation on dietary nutrition, hygiene and behavioral measures (hand disinfection, avoidance of communal facilities, possibly a ban on working in the catering / food industry), if necessary the prescription of antidiarrheal drugs and, if necessary, antiemetics and probiotics .
Loperamide is recommended by the German Society for General Medicine and Family Medicine as symptomatic reliever medication for acute diarrhea. Loperamide is taken orally and is considered effective in the symptomatic treatment of diarrheal diseases of various causes, including: acute, non-specific diarrhea (diarrhea), traveler's diarrhea, diarrhea due to motility disorders and diarrhea associated with irritable bowel syndrome. Other areas of application under medical supervision include diarrhea caused by chemotherapy or protease inhibitors.
Some combination preparations for acute diarrhea with cramps also contain the active ingredient simeticon to relieve gas-related discomfort in the abdomen.
pharmacology
effect
Loperamide (loperamide hydrochloride) acts as an agonist on the µ-opioid receptors in the myenteric plexus (Auerbach plexus). The myenteric plexus runs through almost the entire gastrointestinal tract and is located in the tunica muscularis between the longitudinal and circular muscles of the intestinal wall. In contrast to other, often strongly centrally active opioids and opiates, loperamide mainly acts locally in the intestine, so that at therapeutic doses there are no serious side effects in the nervous system that are known for opioids; d. H. no analgesic effect, no cough suppression, no respiratory depression , no miosis . Likewise, the psychotropic effects known from opioids and opiates are absent; like euphoria, anxiety suppression, or sedation. The absence of these effects also explains why finished medicinal products containing loperamide are not subject to any prescription, let alone BTM prescription. Nevertheless, reports appeared in the specialist press about the misuse of the drug as an intoxicating agent, which, however, is only possible, for example, through very high doses or previous, additive intake of p-GP inhibitors.
Loperamide reduces the activity of the longitudinal and circular smooth muscles in the wall of the small intestine. This results in its antidiarrheal effect: the transit of the intestinal contents in the small intestine is slowed down, so that the intestinal contents have contact with the intestinal mucosa for a longer period of time, so that more fluid and electrolytes can be absorbed from the intestine. An increase in the tone of the anal sphincter muscle was also observed with the use of loperamide , which leads to an improvement in stool continence in patients with and without diarrhea.
Contraindications
The mode of action of loperamide therefore determines when loperamide should not be used: For the primary treatment of infectious diarrheal diseases ( dysentery ), which are often characterized by blood, mucus or pus in the stool, as the immobilization of the intestine causes the pathogens to be excreted with delay depending on the pathogen, further toxins ( toxins are submitted).
Systemic vascular disease can be triggered ( hemolytic-uremic syndrome , HUS), in particular with an infection by enterohaemorrhagic E. coli ( EHEC ) , so loperamide must not be used for treatment. Furthermore, loperamide must not be used in acute attacks of ulcerative colitis, pseudomembranous colitis due to the intake of broad-spectrum antibiotics and in patients in whom inhibition of peristalsis should be avoided because of possible secondary diseases including ileus, megacolon, toxic megacolon.
compatibility
Loperamide is generally well tolerated at the recommended dosage and duration. If acute diarrhea does not improve within 48 hours, a doctor should be informed. A prolonged use of loperamide may only take place under medical prescription and follow-up monitoring. The most common side effects are related to the effect on the dysmotility (abdominal pain, bloated stomach, bloated stomach, nausea, constipation).
Interactions with other drugs
Loperamide is one of the opioids and is structurally related to the analgesics of the pethidine and methadone series. Loperamide, however, is a so-called sham opioid, as it hardly shows any effect on the central opioid receptors , as it normally cannot reach them. Older doctrines attribute this to the inability of loperamide to cross the intact blood-brain barrier. However, there is increasing evidence that it crosses the blood-brain barrier as well as all opioids, but is immediately transported back to the periphery from the central nervous system by means of transport proteins ( P-glycoprotein , PGP) .
A defective blood-brain barrier can lead to far-reaching side effects. In dogs, more precisely in the Collie and related dog breeds, it became known that loperamide can lead to death in connection with the MDR1 defect , which leads to a deficient or absent synthesis of the P-glycoprotein. Such a defect is also known in humans, although there are no known cases with a fatal outcome due to therapeutic loperamide doses. However, medication with loperamide should be avoided in children under the age of 12, as the blood-brain barrier is not yet fully developed, especially in infants.
Loperamide can interact with other drugs and show effects in the central nervous system. If quinidine , verapamil or ketoconazole are taken at the same time , signs of respiratory depression can be triggered. Interactions have also been observed with ritonavir (HIV proteinase inhibitor). Excessive consumption of quinine-containing lemonade (tonic water) in connection with the intake of loperamide can lead to respiratory failure. Loperamide also acts as a FIASMA (functional inhibitor of acid sphingomyelinase ).
Derived connections
Trade names
Binaldan (CH), Enterobe (A), Imodium (A, CH), Imodium acute capsule / lingual / soft capsule (D, A), Lopedium (D), Lopedium acute (D), Lopimed (CH), normakut (A) , numerous generics (D, CH).
Imodium Duo (CH), Imodium N Duo (D)
Individual evidence
- ↑ External identifiers or database links for loperamide hydrochloride: CAS number: 34552-83-5, EC number: 252-082-4, ECHA InfoCard: 100.047.333 , PubChem : 71420 , ChemSpider : 64510 , DrugBank : DBSALT000709 , Wikidata : Q27107231 .
- ↑ a b c d Entry on loperamide. In: Römpp Online . Georg Thieme Verlag, accessed on July 13, 2019.
- ↑ a b Datasheet Loperamide hydrochloride from Sigma-Aldrich , accessed on May 8, 2017 ( PDF ).
- ↑ a b c d e German Society for General Medicine and Family Medicine. DEGAM S1 recommendation for action: "Acute diarrhea - epidemiology, diagnostic and therapeutic recommendations". ( Memento from October 29, 2015 in the Internet Archive ) Status: 09/2013. Valid until 09/2018.
- ^ A b D. E. Baker: Loperamide: a pharmacological review. In: Rev Gastroenterol Disord. 7 Suppl 3, 2007, pp. 11-18 PMID 18192961 .
- ^ World Health Organization: WHO Model List of Essential Medicines 19th List. April 2015, Amended August 2015. Retrieved August 31, 2015.
- ^ Klaus Florey: Profiles of Drug Substances, Excipients and Related Methodology . Volume 19. Academic Press, 1991, ISBN 0-08-086114-8 , p. 342 (online)
- ↑ RA Stokbroekx, J. Vandenberk, AH Van Heertum, GM Van Laar, MJ Van der Aa, WF Van Bever, PA Janssen: Synthetic antidiarrheal agents. 2,2-Diphenyl-4- (4'-aryl-4'-hydroxypiperidino) butyramides. In: J Med Chem. 16 (7), Jul 1973, pp. 782-786 PMID 4725924 .
- ^ Wikipedia: Janssen Pharmaceutica. Retrieved September 9, 2015.
- ↑ a b c d S. B. Hanauer: The role of loperamide in gastrointestinal disorders. In: Rev Gastroenterol Disord. 8 (1), Winter 2008, pp. 15-20. PMID 18477966 .
- ^ Avoxa - Mediengruppe Deutscher Apotheker GmbH: Pharmaceutical newspaper online: watch out for Loperamid. Retrieved August 18, 2018 .
- ↑ A. Lubasch, H. Lode: Importance of antibiotic therapy in infectious enteritis. In: Internist. (Springer-Verlag) 41, 2000, pp. 494-497.
- ↑ WF Caspary: Symptomatic therapy of diarrhea. In: Wolfgang F. Caspary et al. (Hrsg.): Infectiology of the gastrointestinal tract. Springer-Verlag, 2006, ISBN 3-540-41359-6 .
- ↑ Tae-Eun Kim, Howard Lee, Kyoung Soo Lim, SeungHwan Lee, Seo-Hyun Yoon: Effects of HM30181, a P-glycoprotein inhibitor, on the pharmacokinetics and pharmacodynamics of loperamide in healthy volunteers . In: British Journal of Clinical Pharmacology . tape 78 , no. 3 , September 2014, p. 556-564 , doi : 10.1111 / bcp.12368 , PMID 24602137 , PMC 4243906 (free full text).
- ↑ Technical information Loperamide-CT 2mg hard capsules. As of October 2008 (online RTF; 53 kB).
- ↑ Anesthesia and Intensive Care Medicine - 5/2018. Archived from the original on October 31, 2018 ; accessed on October 31, 2018 .
- ↑ J. Kornhuber, M. Muehlbacher, S. Trapp, S. Pechmann, A. Friedl, M. Reichel, C. Mühle, L. Terfloth, T. Groemer, G. Spitzer, K. Liedl, E. Gulbins, P Tripal: Identification of novel functional inhibitors of acid sphingomyelinase. In: PLoS ONE. 6, No. 8, 2011, p. E23852. doi: 10.1371 / journal.pone.0023852 .
- ↑ External identifiers or database links for loperamide N-oxide : CAS number: 106900-12-3, EC number: 600-785-5, ECHA InfoCard: 100.129.450 , PubChem : 71421 , ChemSpider : 16736800 , Wikidata : Q27284849 .