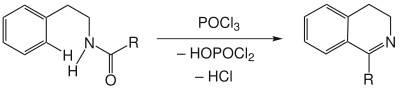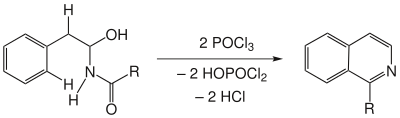Bischler-Napieralski reaction
The Bischler-Napieralski reaction is a reaction from the field of organic chemistry , which is used to produce heterocycles . It is named after its discoverers, the chemists August Bischler (1865–1957) and Bernard Napieralski (formerly employed at the University of Zurich ) and describes the cyclization of amides in the presence of Lewis acids . This reaction is used more often in the total synthesis of alkaloids.
Reaction equation
Amides are cyclized by the action of POCl 3 with elimination of water . However, this elimination of water is purely formal; in fact, HOPOCl 2 and HCl are formed alongside the desired product .
If the aryl groups are less nucleophilic , more drastic reaction conditions must be used. Indoles already react when heated, while benzene requires higher temperatures. POCl 3 is the most common dehydrating agent in chemical reactions. PCl 5 , polyphosphoric acid and ZnCl 2 were also successfully tested.
mechanism
There are two different descriptions in the literature of the mechanism of the Bischler-Napieralski reaction. Which mechanism takes place in a particular case depends, among other things, on the reaction conditions. The difference between the two possibilities lies in the point in time at which the carbonyl oxygen atom is eliminated . In mechanism I it takes place after ring closure with formation of the imine , in mechanism II it takes place with formation of a nitrilium ion before ring closure.
Mechanism I:
Mechanism II:
variant
Contains the starting substance in α -position to the amino group, a hydroxy group , then enters a further formal elimination of water and it forms an isoquinoline - derivative .
The Bischler-Napieralski reaction is important for the total synthesis of certain alkaloids (e.g. papaverine or reserpine ).
See also
swell
- Hans Rudolf Christen : Fundamentals of organic chemistry . Publishers Sauerländer · Diesterweg · Salle , 1977, S. 658th
Individual evidence
- ↑ August Bischler, Bernard Napieralski: To the knowledge of a new isoquinoline synthesis . In: Reports of the German Chemical Society . Vol. 26, No. 2 , 1893, p. 1903 , doi : 10.1002 / cber.189302602143 ( bnf.fr ).
- ^ Wilson M. Whaley, Tuticorin R. Govindachari: The Preparation of 3,4-Dihydroisoquinolines and Related Compounds by the Bischler-Napieralski Reaction . In: Organic Reactions . John Wiley & Sons, Inc., 2004, ISBN 978-0-471-26418-7 .
- ^ FW Bergstrom: Heterocyclic Nitrogen Compounds. Part IIA. Hexacyclic Compounds: Pyridines, Quinolines, and Isoquinolines. In: Chemical Reviews . tape 35 , no. 2 , October 1944, p. 77-277, here p. 218 , doi : 10.1021 / cr60111a001 .
- ^ Gábor Fodor, J. Gal, BA Phillips: The Mechanism of the Bischler-Napieralski Reaction . In: Angewandte Chemie International Edition in English . Vol. 11, No. 10 , 1972, p. 919-920 , doi : 10.1002 / anie.197209191 .
- ↑ a b László Kürti , Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis - Elsevier Academic Press, Burlington / San Diego / London 2005, ISBN 0-12-369483-3 .
- ↑ G. Fodor, S. Nagubandi: Correlation of the von Braun, Knight, Bishler-Napieralski, Beckmann and Schmidt reactions via nitrilium salt intermediates . In: Tetrahedron . tape 36 , no. 10 , 1980, pp. 1279-1300 , doi : 10.1016 / 0040-4020 (80) 85039-3 .