Catechol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Catechol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 6 H 6 O 2 | |||||||||||||||||||||
| Brief description |
colorless crystals |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 110.11 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.34 g cm −3 |
|||||||||||||||||||||
| Melting point |
105 ° C |
|||||||||||||||||||||
| boiling point |
245 ° C |
|||||||||||||||||||||
| Vapor pressure |
20 Pa (20 ° C) |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
Switzerland: 5 ml m −3 or 23 mg m −3 |
|||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Pyrocatechol ( 1,2-dihydroxybenzene ) is a dihydric phenol , an aromatic alcohol . It has two adjacent hydroxyl groups in what is known as the ortho position on a benzene ring .
The English name is catechol (short form of pyrocatechol ), according to IUPAC it is also called 1,2-dihydroxybenzene or benzene-1,2-diol .
In addition to catechol (1,2-dihydroxybenzene), there are two other positionally isomeric forms, namely resorcinol (1,3-dihydroxybenzene) and hydroquinone (1,4-dihydroxybenzene).
History and occurrence
The common name of the chemical compound goes back to the gerber acacia ( Acacia catechu ). It was first isolated from their plant sap , which contains catechin , in 1839 by the chemist K. Reinsch using dry distillation , so-called pyrolysis (ancient name for pyrolysis ). In addition, the terms pyrocatechol and pyrocatechol are common for catechol .
Pyrocatechol is widespread in the plant kingdom and can be obtained by pyrolysis ( pyrolysis reaction) of natural raw materials such as wood, coal and lignin . It is mostly in tree sap and beech wood tar; in the past it was also extracted from the smoldering waters of lignite .
Catechol is also found in significant amounts in tobacco smoke (depending on the brand, 20–500 µg per cigarette). There, catechol is also assigned a role in the tumor-promoting effect of tobacco smoke. The translocation of PKC and the formation of 8-hydroxydeoxyguanosine have been suggested as possible mechanisms .
Extraction and presentation
Pyrocatechol can be prepared with the help of an alkali melt of o -chlorophenol or o -phenolsulfonic acid .
Also by hydrolysis of 2-chlorophenol with sodium hydroxide solution at a higher temperature in the presence of copper sulfate , also in the presence of barium hydroxide and copper (I) chloride or by the oxidative degradation of salicylaldehyde with hydrogen peroxide and sodium hydroxide solution ( Dakin reaction ). An ether cleavage of guaiacol with hydrogen bromide is also possible.
properties
Catechol forms colorless crystals that are easily volatile with water vapor. In air and when exposed to light, it becomes unstable and oxidizes to 1,2-benzoquinone ( auto-oxidation ).
The aqueous solution is colorless, but at pH values above 7 it quickly turns brown due to oxidation in the presence of oxygen . In a neutral solution, the combination with iron (III) chloride results in a green color. This reaction can be used to differentiate the dihydroxybenzenes : Resorcinol gives a violet color, hydroquinone a blue color, which disappears after a short time because the hydroquinone is oxidized by the ferric chloride to p -benzoquinone , which does not show any color reaction. With lead (II) acetate , on the other hand, a colorless precipitate forms. The reaction of catechol with "vanadium sulfuric acid" → mandelin reagent gives a blue-green color . The reaction with chlorinated lime also gives a blue-green color . Pyrocatechol is a powerful reducing agent that can reduce Fehling's solution , Benedict's reagent and ammoniacal silver nitrate solution .
use
The possible uses of catechol are in photo technology as a developer . It is used as an antioxidant and disinfectant . In organic synthesis , it plays a role as a starting material for dyes , fragrances and pharmaceuticals and as a protective group for carbonyl compounds . About 50% of the synthetic catechol produced is used to make pesticides , with the remainder being used as a precursor to chemicals used in making perfumes and medicines.
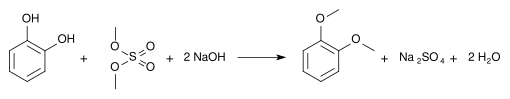
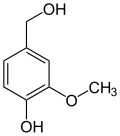
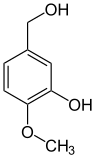
The hydroboration agent catecholborane is a derivative of catechol. Methylations result in guaiacol (monomethyl ether ) and veratrole (dimethyl ether):
Natural substances
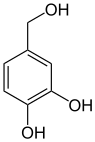
Numerous natural substances are derived from catechol and its methyl ethers guaiacol and veratrol , including the well-known vanillin . The aforementioned three basic compounds are formed by decarboxylation from the corresponding acids: z. B. Veratrol from veratrum acid . Veratrumaldehyde is in turn prepared from Veratrol by means of Vilsmeier formylation .
proof
For qualitative analytical evidence, bromination with potassium bromide and bromine produces the tetrabromo derivative with a melting point of 192 ° C.
safety instructions
The dihydroxybenzenes are irritating to the eyes , skin and respiratory tract . They are harmful to health in contact with the skin and if swallowed . Pyrocatechol is toxic to aquatic organisms and is therefore classified as environmentally hazardous in water hazard class 2 . At the suggestion of the French chemicals authority, the chemical classification of catechol was revised in 2015 and 2016. The Committee for Risk Assessment (RAC) of the European Chemicals Agency (ECHA) decided the classification for catechol as follows on September 16, 2016: catechol is classified as carcinogenic Carc 1B, Muta 2 and Acute Tox 3 for oral and dermal administration. The warnings have been set to H301, H311, H341 and H350. The classifications as Skin Irrit 2 and Eye Irrit 2 were retained with the corresponding warnings H315 and H319. This classification of the RAC has yet to be implemented by the EU Commission into applicable law, but with the publication it represents the state of knowledge that must be taken into account by companies and authorities.
toxicology
Like other dihydroxybenzenes, catechol is absorbed in the gastrointestinal tract and excreted freely in the urine as monosulfate or as monoglucorinide. In addition, absorption through the skin was observed. In the metabolism, 1,4-benzoquinone , trihydroxybenzenes and certain semiquinones are formed as intermediate products .
When catechol is absorbed through the skin, the symptoms observed are similar to those of phenol poisoning . Contact dermatitis also developed in individual cases . In certain cell lines, catechol induced the apoptosis of cells. In erythrocytes , catechol can cause hemolysis .
On a molecular level, it is believed that catechol, through the formation of quinones and other radicals, in combination with a depletion of antioxidants such as glutathione , causes an overall decrease in antioxidant capacity and thus leads to oxidative stress . This could be the cause of the induction of DNA damage . In addition, catechol can interact with cysteine residues and thus possibly lead to the aggregation of proteins or the formation of disulfide bridges .
Individual evidence
- ↑ a b c d e f g h Entry on catechol in the GESTIS substance database of the IFA , accessed on November 15, 2019(JavaScript required) .
- ↑ Entry on catechol. In: Römpp Online . Georg Thieme Verlag, accessed December 10, 2014.
- ↑ Entry on pyrocatechol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on November 15, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 120-80-9 or catechol ), accessed on November 2, 2015.
- ↑ Entry on catechol. In: Römpp Online . Georg Thieme Verlag, accessed on August 2, 2018.
- ↑ Schwelwasser on universal_lexikon.deacademic.com, accessed on August 1, 2016.
- ↑ a b Hans R. Schweizer: Artificial organic dyes and their intermediate products. Springer-Verlag, March 13, 2013, ISBN 978-3-642-87245-7 , p. 142.
- ↑ JD Mold, MP Peyton, RE Means, TB Walker: Determination of catechol in cigarette smoke . In: Analyst . tape 91 , no. 1080 , January 1, 1966, ISSN 1364-5528 , p. 189-194 , doi : 10.1039 / AN9669100189 .
- ↑ Klaus D. Brunnemann, Herng-Cherng Lee, Dietrich Hoffmann: Chemical Studies on Tobacco Smoke. XLVII. On the Quantitative Analysis of Catechols and Their Reduction . In: Analytical Letters . tape 9 , no. October 10 , 1976, ISSN 0003-2719 , pp. 939-955 , doi : 10.1080 / 00032717608059158 .
- ^ R. Gopalakrishna, ZH Chen, U. Gundimeda: Tobacco smoke tumor promoters, catechol and hydroquinone, induce oxidative regulation of protein kinase C and influence invasion and metastasis of lung carcinoma cells . In: Proceedings of the National Academy of Sciences . tape 91 , no. 25 , December 6, 1994, ISSN 0027-8424 , p. 12233-12237 , doi : 10.1073 / pnas.91.25.12233 , PMID 7991611 , PMC 45411 (free full text).
- ↑ Per Leanderson, Christer Tagesson: Cigarette smoke-induced DNA damage: Role of hydroquinone and catechol in the formation of the oxidative DNA adduct, 8-hydroxydeoxyguanosine . In: Chemico-Biological Interactions . tape 75 , no. 1 , 1990, p. 71-81 , doi : 10.1016 / 0009-2797 (90) 90023-G .
- ↑ Alkalischmelze on Spektrum.de, accessed August 1, 2016.
- ↑ Catechol on Spektrum.de, accessed August 1, 2016.
- ↑ Uni Regensburg: Test instructions for the detection of dihydroxybenzenes (Peter Keusch).
- ↑ KF Mandelin, St. Petersburg: About vanadium sulfuric acid, a new reagent for alkoloids. E. Wienecke, 1883.
- ↑ a b Joseph Schomacker: Contribution to the forensic-chemical detection of resorcinol and catechol in the animal body (PDF; 1.3 MB), dissertation, 1886, University of Dorpat.
- ↑ Fiegel, Helmut include: phenol derivatives. In: Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, June 15, 2000, doi : 10.1002 / 14356007.a19_313 .
- ↑ External identifiers or database links for catecholborane : CAS number: 274-07-7, EC number: 205-991-5, ECHA InfoCard: 100.005.447 , PubChem : 67506 , ChemSpider : 10617125 , Wikidata : Q730606 .
- ^ Association of authors: Organikum . 19th edition. Johann Ambrosius Barth, Leipzig / Berlin / Heidelberg 1993, ISBN 3-335-00343-8 , p. 345.
- ^ Association of authors: Organikum . 19th edition. Johann Ambrosius Barth, Leipzig / Berlin / Heidelberg 1993, ISBN 3-335-00343-8 , p. 331.
- ^ Association of authors: Organikum . 19th edition. Johann Ambrosius Barth, Leipzig / Berlin / Heidelberg 1993, ISBN 3-335-00343-8 , p. 653.
- ↑ RAC decision of September 16, 2016.
- ^ GA Garton, RT Williams: Studies in detoxication. 17. The fate of catechol in the rabbit and the characterization of catechol monoglucuronide . In: Biochemical Journal . tape 43 , no. 2 , January 1, 1948, ISSN 0006-2936 , p. 206-211 , doi : 10.1042 / bj0430206 .
- ↑ a b c International Agency for Research on Cancer .: Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide . World Health Organization, International Agency for Research on Cancer, [Lyon] 1999, ISBN 978-92-832-1271-3 .
- ↑ Some fumigants, the herbicides 2,4-D and 2,4,5-T, chlorinated dibenzodioxins and miscellaneous industrial chemicals. In: IARC monographs on the evaluation of the carcinogenic risk of chemicals to man. Vol. 15, Aug. 1977, pp. 1-354, PMID 330387 (Review).
- ↑ JL Moran, D. Siegel, XM Sun, D. Ross: Induction of apoptosis by benzene metabolites in HL60 and CD34 + human bone marrow progenitor cells. In: Molecular pharmacology. Volume 50, Number 3, September 1996, pp. 610-615, PMID 8794901 .
- ↑ B. Bukowska, S. Kowalska: Phenol and catechol induce prehemolytic and hemolytic changes in human erythrocytes. In: Toxicology letters. Volume 152, Number 1, August 2004, pp. 73-84, doi : 10.1016 / j.toxlet.2004.03.025 , PMID 15294349 .
- ↑ George Barreto, Diego Madureira, Francisco Capani, Laura Aon-Bertolino, Ezequiel Saraceno: The role of catechols and free radicals in benzene toxicity: An oxidative DNA damage pathway . In: Environmental and Molecular Mutagenesis . tape 50 , no. 9 , December 2009, p. 771-780 , doi : 10.1002 / em.20500 .
- ↑ U. Stenius, M. Warholm, A. Rannug, S. Walles, I. Lundberg, J. Högberg: The role of GSH depletion and toxicity in hydroquinone-induced development of enzyme-altered foci. In: Carcinogenesis. Volume 10, Number 3, March 1989, pp. 593-599, doi : 10.1093 / carcin / 10.3.593 , PMID 2564322 .
- ^ S. Oikawa: Site specificity and mechanism of oxidative DNA damage induced by carcinogenic catechol . In: Carcinogenesis . tape 22 , no. 8 , August 1, 2001, p. 1239-1245 , doi : 10.1093 / carcin / 8/22/1239 .
- ↑ Nina Schweigert, Alexander JB Zehnder, Rik IL Eggen: Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Mini review . In: Environmental Microbiology . tape 3 , no. 2 , February 2001, ISSN 1462-2912 , p. 81-91 , doi : 10.1046 / j.1462-2920.2001.00176.x .