UV / VIS spectroscopy
The UV / VIS spectroscopy is an optical molecular spectroscopy belonging spectroscopic method , the electromagnetic waves of the ultraviolet (UV) and visible ( English visible , VIS) light utilized. The method is also known as UV / VIS spectrophotometry or electron absorption spectroscopy . In everyday life, the devices used are often imprecisely referred to as photometers .

principle
Light absorption in the range of visible and ultraviolet radiation is caused by electron transitions between different states in the molecule . During these transitions, valence electrons (for example those of the p and d orbitals of the outer shells) are excited, that is, they are raised to a higher energy level.
For example , in order to raise an electron from an occupied ( HOMO ) to an unoccupied, higher orbital ( LUMO ), the energy of the absorbed photon must correspond exactly to the energy difference between the two energy levels. About the context
- Δ
, the wavelength of the absorbed light are calculated for the expended energy, the energy , the Planck constant , the speed of light , the frequency and the wavelength are of the electromagnetic wave. This relationship is sometimes referred to as the Einstein-Bohr equation. This relationship can be roughly represented in simplified form in the form of a tailored size equation :
Substances that only absorb in the UV range ( <400 nm) appear colorless to the human eye. A substance is called colored if it absorbs radiation in the range of the visible spectrum . This is to be expected both with compounds that have low excitation energies (π-to-π * transitions, conjugated π-electron systems such as the polyenes ) and with inorganic ion complexes with incompletely filled electron levels (example: Cu 2+ - Connections (mostly bluish - greenish) versus colorless Cu + compounds). Connections also appear colored when strongly polarizing interactions exist between neighboring particles, as is the case e.g. B. is the case with yellow AgI . With only one absorption area, the eye perceives the color complementary to the absorbed radiation .
Basically, phenomena of radiation absorption are mainly evaluated in the context of UV / VIS spectroscopy. In its basic structure, a light source emits electromagnetic radiation, which is guided through the sample / analyte via a beam path with mirrors and other components (details below) and then hits a detector. By exciting electrons in the sample, the intensity of the radiation is weakened compared to the original primary beam in corresponding areas. This difference in radiation intensity is plotted against the respective wavelength at which measurements were made and output as a spectrum .
Construction of a two-beam UV / Vis spectrometer
The light source emits ultraviolet, visible and near-infrared light in the wavelength range from about 200 nm to 1100 nm. In the monochromator , the wavelength selected for measurement is first selected, whereupon the light beam falls on the sector mirror. The sector mirror lets the light fall alternately through the measurement solution and through the comparison solution. Both solutions are in so-called cuvettes . The two light beams are received in the detector and the intensities are compared in the amplifier. The amplifier then adjusts the intensity of the light beam from the comparison solution to the intensity of the light beam from the measurement solution by retracting the comb diaphragm. This movement is transferred to a recorder or the measured values are passed on to data processing.
Cell-free UV / VIS spectrometers are increasingly being used to determine the concentration of small sample volumes (less than 2 microliters) of samples with higher concentrations. So-called nanophotometers work with layer thicknesses ranging from 0.04 mm to 2 mm. You do not need any cuvettes, no dilutions and can analyze samples with a volume of only 0.3 µl (with the smallest layer thickness), but have a higher detection limit due to the small layer thickness . A proven technique is based on a compression of the sample, which is therefore independent of the surface tension and evaporation of the sample. This method is used for the analysis of nucleic acids (DNA, RNA, oligonucleotides) and proteins (UV absorption at 280 nm). According to the Lambert-Beer law, there is a relationship between absorption and layer thickness. The absorption values for the different layer thicknesses (0.04 mm to 2 mm) can thus be calculated. Small layer thicknesses act like a virtual dilution of the sample, but can only be used at correspondingly higher concentrations. Therefore, manual dilution of the sample can often be dispensed with entirely.
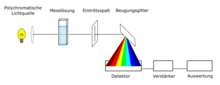
Construction of a diode array UV / VIS spectrometer
Another technology is diode array technology. The sample in the cuvette is irradiated with a light beam, consisting of the continuous wavelength range of the light source (e.g. xenon flash lamp , 190 nm to 1100 nm). The sample absorbs different wavelengths of the light source during a measurement. Unabsorbed light passes through the entrance slit and is split up at a diffraction grating according to its wavelength. The spectrum is detected with the help of a CCD sensor and then evaluated. In the case of non-automated devices, the reference sample must also be measured. The advantages of the technology are short measurement times, as the entire UV / VIS spectrum can be recorded with one measurement, low maintenance costs, as there are no moving components in the spectrometer and the devices can be constructed compactly.
Chemical examples
The π-to-π * transitions are useful for unsaturated hydrocarbons ( e.g. alkenes ). They are made using longer-wave UV light and are easy to measure. Information is obtained about the absorbing wavelength of the molecule, its structure and color . The light absorption takes place in the longer-wave range, the greater the number of conjugated double bonds . If the energy of the π-to-π * transitions is in the range of visible light, the molecule appears colored. It always takes on the complementary color of the light it absorbs.
The following selection rules must always be observed for the electron transitions under consideration (including Laporte's rule ):
- Spin rule: The total spin must be maintained
- Transitions between different spin multiplicities are prohibited. Excitation is only allowed if the total spin of the molecule does not change, i.e. if the same number of paired and unpaired electrons (spins) is present before and after the excitation.
- Ban on transitions with the same parity (Laporte ban)
With the Laporte ban, two queries must be made according to the ligand field theory.
- Does the molecule have an inversion center? If so (e.g. an octahedron), then an excitation is initially not allowed. If not (e.g. a tetrahedron), excitation is permitted.
- Does the parity (sign) of the orbitals change? If so, excitation is allowed (e.g. the transition from s → p orbital). If not, the excitation is not allowed (e.g. the transition from p → f orbital). A transition may only take place from even to odd, or from odd to even (the s and d orbitals are even, the p and f orbitals are odd).
E.g.:
- The transition 3s → 4s is prohibited
- the transition 3s → 4p is allowed
Warning: Prohibited does not mean that these transitions do not occur! The weak color of complexes is caused by vibrations of the ligands relative to the metal center. As a result, the inversion symmetry, which was important in the Laporte ban, is temporarily canceled and a transition can take place.
See also
Web links
- ETH Zurich directory of databases and reference works with UV / VIS spectra
- White paper from Mettler-Toledo: UV / VIS spectrometers in comparison: diode array versus scan technology
Individual evidence
- ^ Manfred Reichenbächer, Jürgen Popp: [ limited preview in the Google book search Structural Analysis of Organic and Inorganic Compounds: An Exercise Book] . Springer Science & Business Media, September 25, 2007, ISBN 978-3-8351-0190-6 , p. 119.
- ^ Christopher G. Morris, Academic Press: Academic Press dictionary of science and technology . Gulf Professional Publishing, 1992, ISBN 0-12-200400-0 , pp. 716 ( limited preview in Google Book search).
- ↑ Collective of authors, lead authors: K. Doernel, R. Geyer: Analytikum - Methods of analytical chemistry and their theoretical foundations . 8th edition. VEB German publishing house for basic industry, Leipzig 1990, ISBN 3-342-00191-7 , p. 259 ff .
- ↑ H. Stranneheim, J. Lundeberg: Stepping stones in DNA sequencing. In: Biotechnology journal. Volume 7, number 9, September 2012, ISSN 1860-7314 , pp. 1063-1073, doi: 10.1002 / biot.201200153 , PMID 22887891 . PMC 3472021 (free full text).
- ↑ PO Michel, C. Degen, M. Hubert, L. Baldi, DL Hacker, FM Wurm: A NanoDrop-based method for rapid determination of viability decline in suspension cultures of animal cells. In: Analytical biochemistry. Volume 430, number 2, November 2012, ISSN 1096-0309 , pp. 138-140, doi: 10.1016 / j.ab.2012.08.028 , PMID 22960013 .
- ↑ MT Kelliher, MS Piraino, ME Gemoules, CA Southern: A comparison of Förster resonance energy transfer analysis approaches for Nanodrop fluorometry. In: Analytical biochemistry. Volume 441, number 1, October 2013, ISSN 1096-0309 , pp. 44-50, doi: 10.1016 / j.ab.2013.06.009 , PMID 23811157 .
- ^ Owen T .: Fundamentals of Modern UV-Visible Spectroscopy: A Primer . 1996.
- ^ Joseph B. Lambert, Scott Gronert, Herbert F. Shurvell, David A. Lightner: Spectroscopy - Structure Clarification in Organic Chemistry 2nd Edition, Pearson Germany, Munich 2012, ISBN 978-3-86894-146-3 , p. 591 -653.