Nitroalkanes
Nitroalkanes (also nitroparaffins ) are chemical compounds consisting of an aliphatic hydrocarbon skeleton are made, in which one or more hydrogen atoms is replaced by a nitro group (-NO 2 are replaced). Depending on the position of the nitro group within the molecule, a distinction is made between primary , secondary and tertiary nitroalkanes.
nomenclature
In the current chemical nomenclature, the prefix nitro- indicates a carbon- bound NO 2 group. However, misleading historical or trivial names are still in use, such as nitroglycerin : Here the alkyl radical is bonded to the nitrogen via a bridging oxygen atom , which is why it is not a nitroalkane, but a triester of nitric acid .
synthesis
In the laboratory, the primary and secondary nitroalkanes are usually obtained by reacting the alkyl iodides with sodium nitrite in dimethylformamide solution in the presence of urea . The tertiary nitroalkanes can be obtained by oxidation of tertiary alkylamines with potassium permanganate .
properties
The melting and boiling points of simple aliphatic nitroalkanes are higher than those of corresponding alkanes or alcohols (see table).
substance Melting point boiling point Methane CH 4 −183 ° C −161 ° C Methanol CH 3 OH −98 ° C 65 ° C Nitromethane CH 3 NO 2 −29 ° C 101 ° C Ethane CH 3 CH 3 −172 ° C −88 ° C Ethanol CH 3 CH 2 OH −114 ° C 78 ° C Nitroethane CH 3 CH 2 NO 2 −90 ° C 114 ° C Propane CH 3 CH 2 CH 3 −188 ° C −42 ° C Propan-1-ol CH 3 CH 2 CH 2 OH −127 ° C 97 ° C 1-nitropropane CH 3 CH 2 CH 2 NO 2 −108 ° C 131 ° C Propan-2-ol CH 3 CH (OH) CH 3 −90 ° C 82 ° C 2-nitropropane CH 3 CH (NO 2 ) CH 3 −93 ° C 120 ° C n -butane CH 3 CH 2 CH 2 CH 3 −138 ° C −0.5 ° C Butan-1-ol CH 3 CH 2 CH 2 CH 2 OH −90 ° C 117 ° C 1-nitrobutane CH 3 CH 2 CH 2 CH 2 NO 2 −81 ° C 152 ° C
substance pKa Nitromethane CH 3 NO 2 10.2 Nitroethane CH 3 CH 2 NO 2 8.6 1-nitropropane CH 3 CH 2 CH 2 NO 2 9 2-nitropropane CH 3 CH (NO 2 ) CH 3 7.74
The nitro group has a negative inductive effect on the carbon atom it carries. Nitroalkanes which contain a hydrogen atom on the α- carbon atom (the carbon atom adjacent to the nitro group ) are CH-acidic compounds. The anion formed during deprotonation can be stabilized both by this inductive effect and by delocalization of the charge by mesomerism . As a consequence of this, primary and secondary nitroalkanes are relatively strongly acidic and can easily be deprotonated in a basic manner. The carbanions formed in this way are reactive intermediates for the synthetically interesting formation of new carbon-carbon bonds.
Reactions
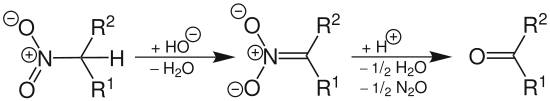
The conversion of nitro groups into carbonyl groups is called the Nef reaction :
This reaction is possible in three ways:
- Acid-base method: the nitronate salt that was previously formed is placed in a strong aqueous acid (standard procedure)
- Oxidative methods
- Reductive methods
See also
- Tetranitromethane , C (NO 2 ) 4
Individual evidence
- ^ Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, p. 949.
- ^ Author collective, Organikum , 16th ed., VEB Deutscher Verlag der Wissenschaften, Berlin, 1986, p. 442.
- ↑ a b c pKa Data Compiled by R. Williams ( Memento of August 1, 2012 in the Internet Archive ) (December 13, 2008).
- ↑ organic-chemistry.org: Nef Reaction (December 13, 2008).