3-methylhexane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
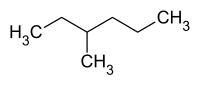
|
||||||||||||||||
| Representation without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 3-methylhexane | |||||||||||||||
| other names |
2-ethylpentane |
|||||||||||||||
| Molecular formula | C 7 H 16 | |||||||||||||||
| Brief description |
highly flammable, volatile liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 100.21 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.69 g cm −3 |
|||||||||||||||
| Melting point |
−119 ° C |
|||||||||||||||
| boiling point |
92 ° C |
|||||||||||||||
| Vapor pressure |
255 h Pa (50 ° C) |
|||||||||||||||
| solubility |
practically insoluble in water (2.6 mg l −1 at 25 ° C) |
|||||||||||||||
| Refractive index |
1.388 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
3-methylhexane is a chemical compound from the group of aliphatic saturated hydrocarbons . It is one of the isomers of n- heptane , has a center of chirality at C-3 and occurs in both enantiomeric forms ( R ) - and ( S ) -3-methylhexane.
use
3-Methylhexane is used as an intermediate in the production of other chemical compounds such as the halogenated derivatives of the compound.
Chirality
3-methylhexane is (together with 2,3-dimethylpentane ) the simplest alkane that exhibits the phenomenon of chirality . At C-3 there is an asymmetric carbon atom whose four substituents are a methyl , an ethyl and a propyl radical , as well as an H atom.
safety instructions
The vapors of 3-methylhexane can form an explosive mixture with air ( flash point approx. −11 ° C, ignition temperature 280 ° C).
literature
- Robert L. Burwell Jr., Howard A. Porte, William M. Hamilton: Racemization, Isomerization and Isotopic Exchange of (+) 3-Methylhexane on a Silica-Alumina Catalyst. In: Journal of the American Chemical Society. 81, 1959, p. 1828, doi : 10.1021 / ja01517a015 .
Individual evidence
- ↑ a b c d e f g h Entry on 3-methylhexane in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ a b Data sheet 3-Methylhexane from Sigma-Aldrich , accessed on July 22, 2010 ( PDF ).



