Neopentane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
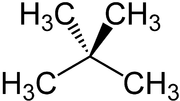
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Neopentane | |||||||||||||||
| other names | ||||||||||||||||
| Molecular formula | C 5 H 12 | |||||||||||||||
| Brief description |
colorless gas |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 72.15 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
0.60 g cm −3 (liquid, at boiling point) |
|||||||||||||||
| Melting point |
−16.6 ° C |
|||||||||||||||
| boiling point |
9.5 ° C |
|||||||||||||||
| Vapor pressure |
0.15 M Pa (20 ° C) |
|||||||||||||||
| solubility |
practically insoluble in water (33 mg l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.3476 (6 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
1000 ml m −3 , 3000 mg m −3 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Neopentane is the common name of 2,2-dimethylpropane , next to n -pentane and isopentane one of the three structural isomers of pentanes with the empirical formula C 5 H 12 . Neopentane is the simplest hydrocarbon with a quaternary carbon atom. At room temperature and atmospheric pressure, neopentane is a colorless, extremely flammable gas. It is sold commercially in liquefied form in pressurized gas cylinders .
Presentation and extraction
Neopentane occurs naturally only in very small quantities in crude oil or natural gas, so that it is not worth extracting from it. The synthesis is achieved by methylating tert-butyl compounds such as tert-butyl iodide using dimethyl zinc or tert-butyl magnesium iodide using dimethyl sulfate , and by hydrolysing neopentyl magnesium chloride using water.
properties
Physical Properties
Compared to the other pentanes, neopentane has a significantly higher melting point at −16.6 ° C. In addition, a polymorphic phase transition with a conversion enthalpy of 2.6305 kJ · mol −1 is observed at −132.7 ° C. Here the transition from the crystalline to a plastic crystalline form takes place. This means that the compound is in a mesomorphic state above this temperature up to the melting point . The behavior is analogous to similar "spherical" molecules such as tetramethylbutane , cubane or adamantane , which form similar mesophases. The compound boils at 9.5 ° C. under normal pressure. It is only very poorly soluble in water at 33 mg / l. The compound is readily soluble in ethanol , diethyl ether , carbon tetrachloride and most aliphatic hydrocarbons .
Thermodynamic properties
According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 3.28533, B = 695.152 and C = −70.679 in the temperature range from 205.6 K to 293.2 K, with A = 3.86373, B = 950.318 and C = −36.329 in the temperature range from 268 K to 313.2 K. or with A = 4.61616, B = 1478, 868 and C = 41,256 in the temperature range from 343 K to 433 K.
| property | Type | Value [unit] | Remarks |
|---|---|---|---|
| Standard enthalpy of formation | Δ f H 0 liquid Δ f H 0 gas |
−190.3 kJ mol −1 −167.9 kJ mol −1 |
as a liquid as a gas |
| Enthalpy of combustion | Δ c H 0 liquid | −3492.4 kJ mol −1 | as a liquid |
| Heat capacity | c p | 163.89 J mol −1 K −1 (5.8 ° C) | as a liquid |
| Triple point | T triple p triple |
256.75 K 268.47 Torr |
|
| Critical temperature | T c | 433.8 K | |
| Critical pressure | p c | 32.0 bar | |
| Critical volume | V c | 0.307 l mol −1 | |
| Critical density | ρ c | 3.26 mol·l −1 | |
| Enthalpy of fusion | Δ f H 0 | 3.26 kJ mol −1 | at the melting point |
| Enthalpy of evaporation | Δ V H 0 | 22.39 kJ mol −1 | at normal pressure boiling point |
The temperature dependence of the evaporation enthalpy can be calculated according to the equation Δ V H 0 = A e (−βT r ) (1 − T r ) β (Δ V H 0 in kJ / mol, T r = (T / T c ) reduced temperature ) with A = 36.76 kJ / mol, β = 0.2813 and T c = 433.8 K in the temperature range between 264 K and 303 K.
Safety-related parameters
The compound forms highly flammable gas-air mixtures. A flash point of less than −7 ° C was determined for liquid neopentane . The explosion range is between 1.3% by volume (40 g / m 3 ) as the lower explosion limit (LEL) and 7.5% by volume (230 g / m 3 ) as the upper explosion limit (UEL). The ignition temperature is 450 ° C. The substance therefore falls into temperature class T2.
Chemical properties
Neopentane is not very reactive. Neopentyl cations caused by the action of acid from neopentyl alcohol are available, go Wagner-Meerwein rearrangements one.
use
The compound can be used as a standard in NMR spectroscopy . Neopentane is rarely used as a component of propellant gases in spray cans or as a refrigerant (R601b).
Web links
Individual evidence
- ↑ a b c d e f g h i j k Entry on neopentane in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-386.
- ↑ Entry on Neopentane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Linde: EC safety data sheet 2,2-dimethylpropane ( Memento of the original from March 4, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , accessed February 3, 2018.
- ↑ a b c d e entry on 2,2-dimethylpropane. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ a b c H. Enokido, T. Shinoda, Y.-I. Mashiko: Thermodynamic Properties of Neopentane from 4K to the Melting Point and Comparison with Spectroscopic Data. In: Bull. Chem. Soc. Yep 42, 1969, pp. 84-91, doi: 10.1246 / bcsj.42.84 .
- ↑ J. Timmermans: Plastic crystals: A historical review. In: J. Phys. Chem. Solids . Volume 18, No. 1, 1961, pp. 1-8, doi: 10.1016 / 0022-3697 (61) 90076-2 .
- ↑ A. Hopfner, N. Parekh, Ch. Horner, A. Abdel-Hamid: The vapor pressure isotope effect of deuterated neopentanes. In: Ber. Bunsen-Ges. Phys. Chem. Vol. 79, 1975, pp. 216-222.
- ↑ AG Osborn, DR Douslin: Vapor Pressure Relations for 15 Hydrocarbons. In: J. Chem. Eng. Data . Volume 19, No. 2, 1974, pp. 114-117, doi: 10.1021 / je60061a022 .
- ^ PP Dawson, Jr., IH Silberberg, JJ McKetta: Volumetric Behavior, Vapor Pressures, and Critical Properties of Neopentane. In: J. Chem. Eng. Data . Volume 18, 1973, pp. 7-15, doi: 10.1021 / je60056a007 .
- ↑ a b c W. D. Good: The enthalpies of combustion and formation of the isomeric pentanes. In: J. Chem. Thermodyn. Volume 2, 1970, pp. 237-244, doi: 10.1016 / 0021-9614 (70) 90088-1 .
- ↑ JG Aston, GH Messerly: Heat capacities and entropies of organic compounds. II. Thermal and vapor pressure data for tetramethylmethane from 13.22 ° K to the boiling point. The entropy from its Raman spectrum. In: J. Am. Chem. Soc. Volume 58, 1936, pp. 2354-2361, doi: 10.1021 / ja01303a002 .
- ↑ a b c d T. E. Daubert: Vapor-Liquid Critical Properties of Elements and Compounds. 5. Branched Alkanes and Cycloalkanes. In: J. Chem. Eng. Data. 41, 1996, pp. 365-372, doi: 10.1021 / je9501548 .
- ↑ ES Domalski, ED Hearing: Heat Capacities and Entropies of Organic Compounds in the Condensed phase. Volume III. In: J. Phys. Chem. Ref. Data . 25, 1996, pp. 1-525, doi: 10.1063 / 1.555985 .
- ^ A b V. Majer, V. Svoboda: Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation. Blackwell Scientific Publications, Oxford 1985, p. 300.



