Tetramethylbutane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
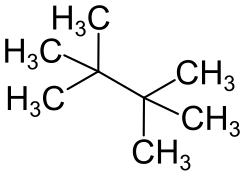
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tetramethylbutane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 18 | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 114.22 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.82 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
100.8 ° C |
|||||||||||||||
| boiling point |
107 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
practically insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tetramethylbutane is the most branched isomer of the octanes . The high degree of branching leads to different physical properties compared to the more chain-like isomers, such as e.g. B. a significantly higher melting point, so that the compound is the only one of the octane isomers at room temperature as a solid.
Presentation and extraction
The synthesis of tetramethylbutane is achieved by linking two tert. -Butyl functions. In an early display variant , tert. -Butylmagnesiumchlorid with tert. -Butyl chloride implemented. The Grignard compound can in a one-pot variant in situ in the implementation of tert. -Butyl chloride are formed and reacted with magnesium .
The dimerization of tert. -Butyl bromide or tert. -Butyl iodide can also be done in the presence of activated copper . Another representation variant is the implementation of tert. -Butyltrichlorosilane with tert. -Butyllithium .
properties
Tetramethylbutane is a solid substance at room temperature that occurs in two polymorphic crystal forms. The crystal form I is present at room temperature and changes to the liquid phase at 100.8 ° C. At −120.6 ° C, a phase transition from crystal form II to form I is observed. Here the transition from the crystalline to a plastic crystalline form takes place. This means that the compound is in a mesomorphic state above this temperature up to the melting point and thus also at room temperature . The behavior is analogous to similar "spherical" molecules such as cubane or adamantane , which form similar mesophases. The boiling point of the compound is already 107 ° C, so that the liquid phase only exists in a temperature range of about 6 K at normal pressure. According to Antoine, the sublimation pressure function results from log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 5.08335, B = 1724.764 and C = −38.383 in the temperature range from 273 K to 338 K. The enthalpies of transformation for the transition from crystal form II to I are Δ tr H = 2.00 kJ mol −1 , for the melt Δ m H = 7.54 kJ mol −1 and the Evaporation Δ b H = 42.91 kJ mol −1 .
Individual evidence
- ↑ a b data sheet 2,2,3,3-tetramethylbutane, ≥94% from Sigma-Aldrich , accessed on March 16, 2013 ( PDF ).
- ↑ a b c d e f g h i Entry on 2,2,3,3-tetramethylbutane in the GESTIS substance database of the IFA , accessed on June 26, 2017(JavaScript required) .
- ↑ a b c d e f g h i D. W. Scott, DR Douslin, ME Gross, GD Oliver, HM Huffman: 2,2,3,3-Tetramethylbutane: Heat capacity, heats of transition, fusion and sublimation, vapor pressure, entropy and thermodynamic functions , in: J. Am. Chem. Soc. , 1952 , 74 (4), pp. 883-887; doi : 10.1021 / ja01124a007 .
- ↑ Entry on 2,2,3,3-tetramethylbutane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Russell E. Marker, Thomas S. Oakwood: Hexamethylethane and Tetraalkylmethanes , in: J. Am. Chem. Soc. , 1938 , 60 (11), p. 2598; doi : 10.1021 / ja01278a011 .
- ↑ Donal T. Flood, George Calingaert: Hexamethylethane , in: J. Am. Chem. Soc. , 1934 , 56 (5), pp. 1211-1212; doi : 10.1021 / ja01320a067 .
- ↑ Francis O. ginah, Thomas A. Donovan Jr., Scott D. Suchan, Deborah R. Pfennig, Greg W. Ebert: Homo Coupling of alkyl Halides and cyclization of α, ω-dihaloalkanes via activated copper , in: J. Org. Chem. , 1990 , 55 (2), pp. 584-589; doi : 10.1021 / jo00289a037 .
- ↑ Michael P. Doyle, Charles T. West: Hindered organosilicon compounds. Synthesis and properties of di-tert-butyl-, di-tert-butylmethyl-, and tri-tert-butylsilanes , in: J. Am. Chem. Soc. , 1975 , 97 (13), pp. 3777-3782; doi : 10.1021 / ja00846a037 .
- ↑ a b E. S. Domalski, ED Hearing: Heat Capacities and Entropies of Organic Compounds in the Condensed phase. Volume III , in: J. Phys. Chem. Ref. Data , 1996 , 25 , pp. 1-525; doi : 10.1063 / 1.555985 .
- ↑ Osborne, NS; Ginnings, DC: Measurements of heat of vaporization and heat capacity of a number of hydrocarbons in J. Res. NBS, 1947 , 39, 453-477.