Tungsten hexacarbonyl
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
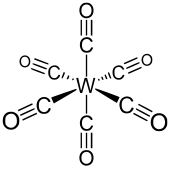
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tungsten hexacarbonyl | |||||||||||||||
| other names |
Tungsten carbonyl |
|||||||||||||||
| Molecular formula | [W (CO) 6 ] | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 351.90 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.65 g cm −3 |
|||||||||||||||
| Sublimation point |
150 ° C (decomposition) |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tungsten hexacarbonyl is a chemical compound with the formula [W (CO) 6 ]. Like its chromium and molybdenum analogues, this colorless compound is a volatile, air-stable complex of tungsten in the zero oxidation state. Tungsten hexacarbonyl was found in trace concentrations in the gaseous vapors of sewage sludge.
presentation
Tungsten hexacarbonyl is produced by the reduction of tungsten hexachloride (WCl 6 ) under carbon monoxide pressure . It is seldom made this way in the laboratory, however, because the equipment required is expensive and the connection is inexpensive to purchase.
In the reductive carbonylation , copper powder or Devarda alloy can also be used as a halide acceptor instead of triethylaluminum .
Properties and structure
Tungsten hexacarbonyl has an octahedral geometry (O h ). The six carbon monoxide ligands are positioned radially around the central tungsten atom. The dipole moment of the complex is 0 Debye . The toilet distance is 207 pm . The wave number of the CO stretching vibration ν CO of the free carbon monoxide is 1998 cm −1 . It is a stable 18-valence electron complex .
The connection is relatively stable in air. It is sparingly soluble in non-polar organic solvents. Like all metal carbonyls , if improperly handled , tungsten hexacarbonyl is a potentially dangerous source of volatile metal and carbon monoxide.
Reactions
The carbon monoxide ligands in tungsten hexacarbonyl can be substituted by other ligands . It behaves similarly to the analogous molybdenum complex , but usually forms kinetically more stable compounds.
A derivative is the dihydrogen complex W (CO) 3 [P (C 6 H 11 ) 3 ] 2 (H 2 ), which was prepared by Kubas in 1982. It is the first known dihydrogen complex, represented by the Hieber base reaction .
Up to three carbon monoxide ligands can be replaced by acetonitrile .
use
Tungsten hexacarbonyl was used for the desulfurization of organosulfur compounds and as a precursor to catalysts for alkene metathesis .
Tungsten hexacarbonyl is used as a precursor in electron beam-induced deposition . Since it evaporates easily and disintegrates with the electron beam , it provides an easily accessible source for tungsten atoms.
Individual evidence
- ↑ a b c Christoph Elschenbroich : Organometallchemie , 6th edition, Teubner, Wiesbaden 2008, ISBN 978-3-8351-0167-8 , p. 330.
- ↑ Data sheet Tungsten hexacarbonyl, ≥99.9% trace metals basis, purified by sublimation from Sigma-Aldrich , accessed on October 23, 2011 ( PDF ).
- ↑ Entry on tungsten hexacarbonyl. In: Römpp Online . Georg Thieme Verlag, accessed on December 13, 2015.
- ↑ Entry on Tungsten hexacarbonyl at ChemBlink , accessed on December 28, 2011.
- ↑ a b Entry on tungsten hexacarbonyl in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ J. Feldmann: Determination of Ni (CO) 4 , Fe (CO) 5 , Mo (CO) 6 , and W (CO) 6 in sewage gas by using cryotrapping gas chromatography inductively coupled plasma mass spectrometry. In: J Environ Monit , 1999, 1: pp. 33-37, doi : 10.1039 / a807277i .
- ↑ Georg Brauer (ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1822.
- ^ GJ Kubas: Metal Dihydrogen and σ-Bond Complexes , Structure, Theory, and Reactivity; Kluwer Academic / Plenum Publishers: New York, 2001, 444 pp., ISBN 978-0-306-46465-2 .
- ↑ Gregory J. Kubas, Lori Stepan Van Der Sluys, Ruth Ann Doyle, Robert J. Angelici: Tricarbonyltris (Nitrile) Complexes of Cr, Mo, and W , Inorganic Syntheses , 1990 , 28 , pp. 29-33, doi : 10.1002 /9780470132593.ch6 .


