Carbene complexes
Carbene complexes are transition metal complexes which formally at least one metal - carbon - double bond included. They are therefore organometallic compounds and are divided into two subgroups - Fischer carbene complexes and Schrock carbene complexes, whereby the electronic environment of the carbon atom bound to the metal is decisive for the classification.
The binding carbon in Fischer carbene complexes is substituted by electron-withdrawing groups and therefore electrophilic . In Schrock-carbene complexes are due to its electron-donating substituent is a nucleophilic carbon atom. The Grubbs catalyst , which is used in olefin metathesis, is an air-stable further development of the Schrock carbene complexes .
history
As early as 1915, Lev Alexandrovich Tschugajew produced the first carbene complex by reacting tetrakis (methyl isocyanide) platinum (II) with hydrazine . However, he did not realize the nature of this connection. The structure of the resulting carbene complex was only clarified in the 1970s. In 1963 M. Ryang converted iron pentacarbonyl with organolithium compounds to form aldehydes and ketones . The formation of the intermediate carbene complexes was not recognized by him.
In 1964, Ernst Otto Fischer converted chromium hexacarbonyl with phenyllithium at the Technical University of Munich . After alkylation with the Meerwein salt trimethyloxonium tetrafluoroborate , he came up with the first proven carbene complex. The complex class was named after its discoverer, Fischer carbene complexes.
Transition metal complexes with stable carbenes ( N-heterocyclic carbenes ) emerged from 1995.
Bond ratio
In Fischer carbene complexes, the free carbene is formally a singlet carbene (both electrons are in an orbital, multiplicity 1). The σ-bonding occurs through a doubly occupied carbene orbital, the π-bonding through the doubly occupied metal orbital .
The bonding relationships can be roughly expressed by the following mesomeric boundary structures:
The bond is polarized, with δ- the metal and δ + the carbon. Since the carbene carbon is positively polarized, it is called an electrophilic carbene.
In Schrock carbene complexes, the free carbene is formally a triplet carbene . This means that the two electrons of the carbene are in two different orbitals, which results in a multiplicity of three. The σ-bond and the π-bond back are thus each formed by an electron of the metal and the ligand.
Fischer carbene complexes
Fischer carbene complexes have a heteroatom on the carbenoid carbon . Fischer carbene complexes are formed with late transition metals in low oxidation states and are electrophilic in character.
presentation
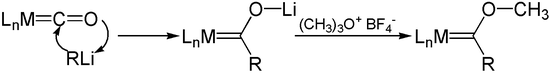
Fischer carbene complexes can easily be obtained by the addition of organometallic organyls to carbonyl complexes and subsequent alkylation . For this purpose, a lithium alkyl can be used, for example, which first adds to the carbonyl ligand . The alkylation is usually carried out with the Meerwein salt trimethyloxonium tetrafluoroborate .
EO Fischer studied the chemistry of the carbene complexes in detail. In addition to the original method of nucleophilic attack on metal carbonyls , he developed other methods. He succeeded in transferring carbene ligands to other complexes by photochemical excitation. By nucleophilic attack on the carbine carbon of a cationic carbine complex with cyanide and rhodanide ions, Fischer succeeded in preparing novel carbene complexes.
Analogous to the Fischer method, carbene complexes can be prepared by nucleophilic attack on isocyanide complexes. Joseph Chatt obtained crystalline carbene complexes by reacting a series of isocyanide complexes of the type [PtCl 2 (PhNC) (PEt 3 )] with ethanol .
The synthetic chemistry of the carbene complexes is diverse. Wolfgang A. Herrmann succeeded in preparing carbene complexes by converting half-sandwich complexes of manganese with diazo compounds . Ulrich Schöllkopf alkylated mercury acyl complexes and in this way came to carbene complexes. Öfele converted metal carbonylates such as disodium chromopentacarbonylate with 1,1-dichlorocyclopropene derivatives to form the carbene complex pentacarbonyl (2,3-diphenylcyclopropenylidene) chromium (0).
Cardin succeeded in preparing carbene complexes by cleaving a C = C double bond with platinum complexes of the type ((PR 3) PtCl 2 ) 2 . In addition, carbene complexes can be modified, such as the replacement of non-carbene ligands, such as carbon monoxide with a phosphine ligand, or through reactions on the ligand, such as substitutions.
Reactions
The reactions of the Fischer carbene complexes are diverse and can be divided into reactions on the non-carbene ligand, such as its substitution by another ligand, the substitution of the carbene ligand and addition reactions on the carbene carbon. Reactions on the carbene ligand and rearrangements are also possible. Fischer-carbene complexes are used in the Dötz reaction .
Schrock carbene complexes
Schrock carbene complexes are nucleophilic carbene complexes which, in contrast to Fischer carbenes, are not heteroatom-substituted . They are formed with early transition metals in high oxidation states and are named after Richard R. Schrock .
presentation
One way of synthesizing Schrock carbene complexes is given in the following scheme. Tantalum (V) pentachloride is reacted with neopentyllithium . The short-lived intermediate tantalum (V) pentaneopentylate is formed here. This breaks down with the elimination of neopentane to form the Schrock carbene complex.
Since Schrock-carbene complexes are often not very stable, they are often only produced shortly before they are used.
Reactions
Schrock carbene complexes are used for alkylenations. For example the Tebbe reagent for methylenation, i.e. the introduction of a methylene group , of ketones .
literature
- E. Riedel (Ed.): Moderne Anorganische Chemie , de Gruyter, 3rd edition, Berlin 2007, ISBN 978-3-11-019060-1 .
- C. Elschenbroich: Organometallchemie , Teubner, 5th edition, Wiesbaden 2005, ISBN 3-519-53501-7 .
- Karl Dötz: Transition Metal Carbene Complexes , 264 pages, Verlag Chemie, Weinheim, (June 1983) ISBN 0-89573-073-1
- Ernst Otto Fischer : On the way to carbene and carbine complexes (Nobel Lecture) . In: Angewandte Chemie . tape 86 , no. 18 , 1974, p. 651-663 , doi : 10.1002 / anie.19740861802 .
Web links
- José Barluenga: Fischer carbene complexes. A new tool for heterocyclic synthesis. (PDF) In: IUPAC.org. March 6, 2002, accessed August 25, 2012 .
Individual evidence
- ↑ L. Chugaev, M. Skanavy-Grigorieva: J. Russ. Chem. Soc. , 47 (1915), p. 776.
- ^ A. Burke, Alan L. Balch, John H. Enemark: Palladium and platinum complex resulting from the addition of hydrazine to coordinated isocyanide. In: Journal of the American Chemical Society. 92, 1970, pp. 2555-2557, doi: 10.1021 / ja00711a063 .
- ^ A b E. O. Fischer, A. Maasböl: On the question of a tungsten-carbonyl-carbene complex. In: Angewandte Chemie. 76, 1964, pp. 645-645, doi: 10.1002 / anie.19640761405 .
- ^ WA Herrmann, M. Elison, J. Fischer, C. Köcher, GRJ Artus: Metal complexes of N-heterocyclic carbenes — a new structural principle for catalysts in homogeous catalysis. Angew. Chem. Int. Edn Engl. 34: 2371-2374 (1995).
- ↑ EO Fischer, H.-J. Beck: Transfer of the methoxyphenylcarbene ligand from molybdenum to iron and nickel. In: Angewandte Chemie. 82, 1970, pp. 44-45, doi: 10.1002 / anie.19700820109 .
- ↑ Ernst Otto Fischer, Peter Stückler, Fritz Roland Kreißl: transition metal carbene complexes. In: Journal of Organometallic Chemistry. 129, 1977, pp. 197-202, doi: 10.1016 / S0022-328X (00) 92491-4 .
- ↑ EM Badley, J. Chatt, RL Richards, GA Sim: The reactions of isocyanide complexes of platinum (II): a convenient route to carbene complexes. In: Journal of the Chemical Society D: Chemical Communications. 1969, p. 1322, doi: 10.1039 / C29690001322 .
- ↑ Wolfgang Anton Herrmann: A new method for the preparation of transition metal carbene complexes. In: Angewandte Chemie. 86, 1974, pp. 556-557, doi: 10.1002 / anie.19740861508 .
- ↑ U. Schöllkopf, F. Gerhart: Synthesis and reactions of stable mercury-carbene complexes. In: Angewandte Chemie. 79, 1967, pp. 990-990, doi: 10.1002 / anie.19670792232 .
- ↑ K. Öfele: Pentacarbonyl (2,3-diphenylcyclopropenylidene) chromium (0). In: Angewandte Chemie. 80, 1968, pp. 1032-1033, doi: 10.1002 / anie.19680802410 .
- ^ DJ Cardin, B. Cetinkaya, MF Lappert: Transition metal-carbene complexes. In: Chemical Reviews. 72, 1972, pp. 545-574, doi: 10.1021 / cr60279a006 .