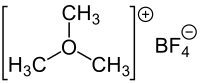
Trimethyloxonium tetrafluoroborate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Trimethyloxonium tetrafluoroborate | ||||||||||||||||||
| Molecular formula | (CH 3 ) 3 O (BF 4 ) | ||||||||||||||||||
| Brief description |
colorless, crystalline solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 147.91 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Sublimation point |
202-203 ° C |
||||||||||||||||||
| solubility |
Decomposes in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Trimethyloxonium tetrafluoroborate , [(CH 3 ) 3 O + BF 4 - ] is a strong alkylating reagent that belongs to the Meerwein salts (named after the German chemist Hans Meerwein ). Occasionally it is also referred to simply as sea wine salt , but this is not clear.
Presentation and extraction
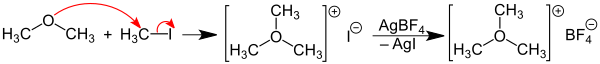
Trimethyloxonium tetrafluoroborate can be obtained by reacting dimethyl ether with methyl iodide and silver tetrafluoroborate . Initially, the ether reacts with methyl iodide to form trimethyloxonium iodide. This is then converted in situ with silver tetrafluoroborate with precipitation of silver iodide to trimethyloxonium tetrafluoroborate.
properties
In trimethyloxonium is a colorless solid at 202-203 ° C sublimated . It reacts violently with water to form an acidic solution .
Reactions
Trimethyloxonium tetrafluoroborate decomposes violently in water to form dimethyl ether, methanol and tetrafluoroboric acid .
It is a very strong methylating agent that can be used, for example, in the synthesis of Fischer carbenes . The lithium compound formed as an intermediate is methylated with trimethyloxonium tetrafluoroborate to form a Fischer carbene.
Individual evidence
- ↑ a b c Data sheet trimethyloxonium tetrafluoroborate (PDF) from Merck , accessed on April 22, 2010.
- ↑ a b Data sheet Trimethyloxonium tetrafluoroborate from Sigma-Aldrich , accessed on April 9, 2011 ( PDF ).
- ↑ GA Olah, H. Doggweiler, JD Felberg: "Onium Ylide Chemistry. 2. Methylenedialkyloxonium Ylides", in: J. Org. Chem. , 1984 , 49 (12) , pp. 2112-2116; doi : 10.1021 / jo00186a006 .
literature
- H. Meerwein: Triethyloxonium Fluoborate In: Organic Syntheses . 46, 1966, p. 113, doi : 10.15227 / orgsyn.046.0113 ; Coll. Vol. 5, 1973, p. 1080 ( PDF ).
- TJ Curphey: Trimethyloxonium Tetrafluoroborate In: Organic Syntheses . 51, 1971, p. 142, doi : 10.15227 / orgsyn.051.0142 ; Coll. Vol. 6, 1988, p. 1019 ( PDF ).

![{\ displaystyle {\ ce {[(CH3) 3O] + [BF4] - + H2O -> (CH3) 2O + CH3OH + HBF4}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/eec7703d18464eaaa225e3ec23a05b48545e4f72)
