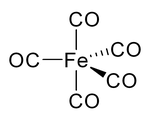
Iron pentacarbonyl
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Iron pentacarbonyl | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | [Fe (CO) 5 ] | |||||||||||||||
| Brief description |
oily yellow to red liquid with a musty odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 195.90 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
|
|||||||||||||||
| Melting point |
−21 ° C |
|||||||||||||||
| boiling point |
105 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
practically insoluble in water |
|||||||||||||||
| Refractive index |
1.453 (22 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 0.1 ml m −3 or 0.8 mg m −3 (calculated as iron) |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Iron pentacarbonyl is a complex compound and next to the diiron nonacarbonyl Fe 2 (CO) 9 and the triiron dodecacarbonyl Fe 3 (CO) 12 it is the simplest of the three known carbonyls of iron . Under standard conditions it is a straw-yellow liquid.
Manufacturing
Technically, iron pentacarbonyl is produced from finely divided iron and carbon monoxide at 150 to 200 ° C under a pressure of 50 to 300 bar. Pure iron without an oxide layer reacts with CO even at room temperature and normal pressure. The purification takes place by means of distillation . It is an equilibrium reaction , the position of which depends heavily on temperature and pressure. The formation of iron pentacarbonyl is exothermic by −226.92 kJ mol −1 .
At high temperatures, the reverse reaction or decomposition becomes relevant, which in addition to carbon monoxide provides a particularly pure iron powder (99.98 to 99.999% iron), the so-called carbonyl iron .
properties
Physical Properties
Iron pentacarbonyl is a yellow to red liquid that boils at 105 ° C under normal pressure . The vapor pressure function results according to Antoine according to log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 5.18943, B = 1960.896 and C = −0.228 in the temperature range from 267 to 378 K.
Compilation of the most important physical properties property Type Value [unit] Remarks Standard enthalpy of formation Δ f H 0 liquid −766.09 kJ mol −1
−3911 kJ kg −1as a liquid Enthalpy of combustion Δ c H 0 liquid −1606 kJ mol −1
−8200 kJ kg −1as a liquid to CO 2 and Fe 2 O 3 Heat capacity c p 235 J mol −1 K −1 (20 ° C)
1.2 J g −1 K −1 (20 ° C)as a liquid Critical temperature T c 285-288 ° C Critical pressure p c 2.90 MPa viscosity η 76 mPas (20 ° C) Thermal conductivity λ 0.139 W m −1 K −1 Linear expansion coefficient α 0.00125 K −1 Enthalpy of fusion Δ F H 13.6 kJ mol −1
69.4 kJ kg −1at the melting point Enthalpy of evaporation Δ V H 37.2 kJ mol −1
190 kJ kg −1at normal pressure boiling point Refractive index n D 22 1.518
Investigations using X-ray diffractometry at −100 ° C showed that the molecule has a trigonal bipyrimidal structure. The compound is completely miscible with petroleum ether , n-hexane , benzene , n-pentanol and other higher alcohols, diethyl ether , acetone , acetic acid and ethyl acetate .
Chemical properties
Iron pentacarbonyl does not react with atmospheric oxygen at room temperature. The compound is stable to water and weak or dilute acids. With concentrated acids, the corresponding salts are formed with the release of carbon monoxide and hydrogen. The corresponding iron halides result from the reactions with halogens. The compound acts as a reducing agent for organic compounds. Nitro aromatics can be reduced to anilines or ketones to alcohols. The Hieber base reaction leads to iron carbonyl salts, such as sodium tetracarbonyl ferrate , which are also strong reducing agents.
Photolysis of the pure compound or its solutions gives the diiron nonacarbonyl. This reaction also takes place when exposed to light in the visible spectral range, so that diiron nonacarbonyl can be formed as an impurity when exposed to daylight.
Safety-related parameters
Iron pentacarbonyl forms highly flammable vapor-air mixtures. The compound has a flash point of −15 ° C. The explosion range is between 3.7 vol.% As the lower explosion limit (LEL) and 12.5 vol.% As the upper explosion limit (UEL). The ignition temperature is 55 ° C.
use
Iron pentacarbonyl is used as a starting material for the synthesis of organometallic iron compounds which, among other things, have versatile catalytic properties.
It used to be used as an anti-knock agent in petrol , for example in Motalin (premium petrol based on the synthetic Leuna petrol in Germany in the 1920s and 1930s). However, this use was abandoned because iron oxides were deposited in the engine and on the spark plugs. With iron pentacarbonyl, 92 RON could have been achieved at an addition concentration of approx. 6.5 g / l.
Iron pentacarbonyl is used to produce semi-transparent iron oxide pigments with a very high chemical purity. Combustion takes place in an excess of atmospheric oxygen at temperatures between 580 ° C and 800 ° C. Orange to red, amorphous products with small grain sizes between 10 and 20 nm result.
Special effect pigments can be produced through controlled combustion in the presence of aluminum powder in a fluidized bed reactor at 450 ° C. Here, the aluminum particles are coated with α – iron (III) oxide, whereby various golden, orange or red shades with high color brilliance can be achieved over different layer thicknesses.
Iron pentacarbonyl served as a rust stopper or rust protection agent in the GDR. It was used to remove rust stains from metallic surfaces or as a primer coat on steel. This was a red-brown, slightly oily liquid with a faint odor.
Individual evidence
- ↑ a b c d e f g h i j k l m n o Entry on iron pentacarbonyl in the GESTIS substance database of the IFA , accessed on November 18, 2017(JavaScript required) .
- ↑ a b c d e f g h i j E. Wildermuth, H. Stark, G. Friedrich, FL Ebenhöch, B. Kühborth, J. Silver, R. Rituper: Iron Compounds. In: Ullmann's Encyclopedia of Technical Chemistry . Wiley-VCH, Weinheim 2012, doi : 10.1002 / 14356007.a14_591 .
- ↑ a b c d Entry on iron carbonyls. In: Römpp Online . Georg Thieme Verlag, accessed on November 19, 2017.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-308.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 13463-40-6 or iron pentacarbonyl ), accessed on November 2, 2015.
- ↑ Wiberg, E .; Wiberg, N .; Holleman, AF : Inorganische Chemie , 103rd edition, 2017 Walter de Gruyter GmbH & Co. KG, Berlin / Boston, ISBN 978-3-11-026932-1 , p. 2119, (accessed from De Gruyter Online).
- ↑ a b M. Bertau, A. Müller, P. Fröhlich, M. Katzberg: Industrielle Inorganische Chemie. 4th edition. John Wiley & Sons, 2013, ISBN 978-3-527-33019-5 , p. 260.
- ^ DR Stull: Vapor Pressure of Pure Substances. Organic and Inorganic Compounds. In: Ind. Eng. Chem. 39, 1947, pp. 517-540, doi: 10.1021 / ie50448a022
- ^ MW Chase, Jr .: NIST-JANAF Themochemical Tables. In: J. Phys. Chem. Ref. Data, Monograph. 9, 1998, 1-1951.
- ↑ a b c d e-EROS Encyclopedia of Reagents for Organic Synthesis . 1999-2013, John Wiley and Sons, entry for Pentacarbonyliron, accessed November 21, 2017.
- ↑ D. Braga, F. Grepioni, AG Orpen: nickel carbonyl [Ni (CO) 4 ] and iron carbonyl [Fe (CO) 5 ]: molecular structures in the solid state. In: Organometallics . 12, 1993, pp. 1481-1483, doi: 10.1021 / om00028a082 .
- ↑ Anti- knock drugs
- ^ A b G. Pfaff: Inorganic Pigments. Walter de Gruyter, Berlin / Boston 2017, ISBN 978-3-11-048450-2 , p. 199 and p. 216.
Web links
- Curves relating to the relationship between additive concentration and achievable RON, accessed on December 23, 2016.



![{\ displaystyle \ mathrm {Fe + 5 \ CO \ \ rightleftharpoons \ [Fe (CO) _ {5}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f3779a7c014440a7d7b8145ccdee156fabddf2bb)




