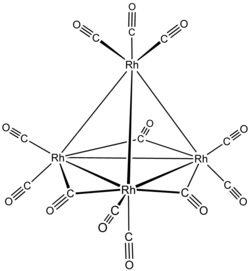
Tetrarhodium dodecacarbonyl
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tetrarhodium dodecacarbonyl | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | [Rh 4 (CO) 12 ] | |||||||||||||||
| Brief description |
orange solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 747.74 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
~ 75 ° C |
|||||||||||||||
| boiling point |
~ 150 ° C (decomposition) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tetrarhodium dodecarbonyl is a complex compound of rhodium from the group of metal carbonyls .
Extraction and presentation
Tetrarhodium dodecarbonyl can be obtained by reacting dirhodium tetracarbonyl dichloride with carbon monoxide .
The synthesis by high pressure carbonylation of anhydrous rhodium (III) chloride at 50-80 ° C in the presence of copper , cadmium or tin as an auxiliary metal is also possible.
properties
Tetrarhodiumdodecarbonyl is an orange to red, air-stable solid that is sparingly soluble in aliphatic solvents and moderately soluble in benzene and diethyl ether . It decomposes when exposed to concentrated acids and bases, in the latter with metal separation. At 100 ° C., decomposition takes place in a nitrogen atmosphere with the formation of hexarhodiumhexadecacarbonyl [Rh 6 (CO) 16 ].
use
Tetrarhodium dodecarbonyl is used as a starting material for the production of rhodium catalysts .
Individual evidence
- ↑ a b c d e data sheet Tetrarhodium dodecacarbonyl from Sigma-Aldrich , accessed on September 12, 2013 ( PDF ).
- ↑ a b c d e Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1836.
- ↑ Jean -Marie Basset, Rinaldo Psaro, Dominique Roberto, Renato Ugo: Modern Surface Organometallic Chemistry . John Wiley & Sons, 2009, ISBN 3-527-62710-3 , pp. 334 ( limited preview in Google Book search).

![{\ displaystyle \ mathrm {2 \ [RhCl (CO) _ {2}] _ {2} +4 \ CO \ longrightarrow [Rh_ {4} (CO) _ {12}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4c97871628e748f349379770ee2d78f784b1efca)